The adherend often establishes ultimate joint durability. The morphology of its surface determines the degree of physical bonding (mechanical interlocking) with the polymer and its chemistry, in part, determines the degree and type of chemical bonding. Furthermore, the stability of the adherend and its surface determines the ultimate limit of durability. Once the adherend becomes degraded, the bondline is reversibly damaged and the joint fails.
Each material exhibits its own form of degradation and conditions under which the degradation occurs. For aluminum adherends, moisture causes hydration of the surface, i. e., the A12O3 that is formed during the surface treatment is transformed into the
|
|
|
|
|
|
![]() Aluminum hydroxide
Aluminum hydroxide
propagation
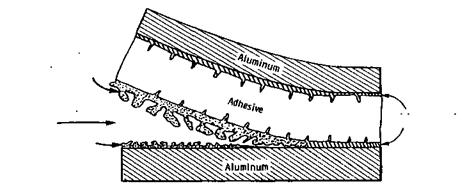
Figure 2 Schematic representation of hydration causing crack propagation in a wedge test specimen. The increase in volume upon hdyration induces stresses at the crack tip that promote crack growth. (From Ref. 4.) oxyhydroxide AlOOH (boehmite) or trihydroxide Al(OH)3 (bayerite). The transformation to the hydroxide results in an expansion of the interphase (the volume occupied by the hydroxide is larger than that originally occupied by the A12O3). This expansion and the corresponding change in surface morphology induce high stresses at the bondline. These stresses, coupled with the poor mechanical strength of the hydroxide, promote crack propagation near the hydroxide/metal interface, as shown schematically in Fig. 2 [1].
The rate of hydration of the aluminum oxide depends on a number of factors, including surface chemistry (treatment), presence of hydration/corrosion inhibitors in the primer or applied to the surface, temperature, and the amount of moisture present at the surface or interface. One surface treatment that provides an oxide coating that is inherently hydration resistant is phosphoric acid anodization (PAA) [30]. Its stability is due to a layer of phosphate incorporated into the outer Al2O3 surface during anodization; only when this phosphate layer goes into solution does the underlying Al2O3 hydrate to AlOOH [35]. The hydration process is illustrated in the surface behavior diagram of Fig. 3 [35,36]. It shows hydration to occur in three stages: (I) a reversible adsorption of water, (II) slow dissolution of the phosphate layer followed by rapid hydration of the freshly exposed Al2O3 to AlOOH, and (III) further hydration of AlOOH to Al(OH)3.
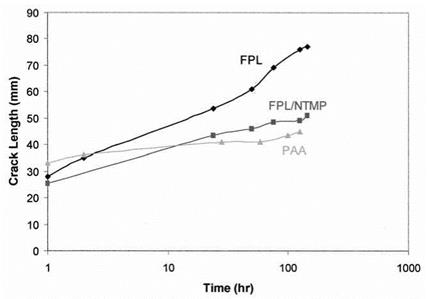
Another means of providing a hydration-resistant surface is its treatment with a hydration inhibitor [37]. Figure 4 shows wedge tests results for a Forest Product Laboratory (FPL) bond, [38] an FPL bond pretreated with nitrilotrismethylenephos — phonic (NTMP) acid [39-41], and a PAA bond. The monolayer coverage of NTMP stabilizes the surface against hydration and provides wedge test bond performance similar to that of PAA-treated adherends.
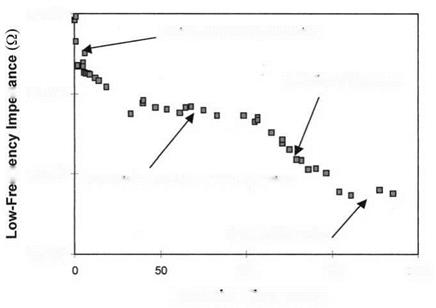
Although the evolution of surface chemistry depicts the hydration of bare surfaces, the same process occurs for buried interfaces within an adhesive bond. This was first demonstrated by using electrochemical impedance spectroscopy (EIS) on an adhesive — covered FPL aluminum adherend immersed in hot water for several months [42]. The EIS, which is commonly used to study paint degradation and substrate corrosion [43,44], showed absorption of moisture by the epoxy adhesive and subsequent hydration of the underlying aluminum oxide after 100 days (Fig. 5). At the end of the experiment, aluminum hydroxide had erupted through the adhesive.
|
|
|
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|
||
|
|||
![]()
Figure 5 Low-frequency electrochemical impedance of an epoxy-coated FPL aluminum adherend as a function of immersion time in 50°C water. (From Ref. 42.)
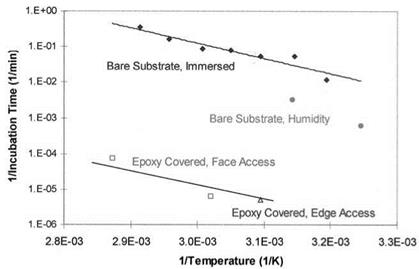
Subsequent investigations showed that identical hydration reactions occurred on both bare aluminum surfaces as well as bonded surfaces, but at very different rates of hydration [45]. An Arrhenius plot of incubation times prior to hydration of bare and buried FPL surfaces clearly showed that the hydration process exhibited the same energy of activation (~82 kJ/mol) regardless of the bare or covered nature of the surface (Fig. 6). On the other hand, the rate of hydration varies dramatically, depending on the concentration of moisture available to react at the oxide/polymer interface or the oxide surface. The epoxy-covered surfaces have incubation times (and rate constants) three to four orders of magnitude longer than bare, immersed specimens, reflecting the limited amount of moisture absorbed by the epoxy and free to react with the oxide.
Steel adherends are also subject to corrosion in moist environments. Unfortunately, no general etch or anodization treatment has been developed that provides superior bond durability [12,18,46]. In part, this is due to the lack of a coherent, adherent stable oxide— iron oxides do not protect the underlying substrate from the environment. Equally important, the different steel metallurgies react to chemical treatments differently—a procedure that may give good results for one steel alloy may give very poor results for another, similar steel alloy.
In contrast to aluminum and titanium structural bonds where performance can be optimized for most aerospace applications, steel bonds are often designed to minimize cost as long as certain performance standards are met [47]. If feasible, many manufacturers prefer to select adhesives or primers that provide adequate strength and durability with untreated steel rather than to prepare the surface for bonding.
The most common surface treatments are grit blasting or other mechanical abrasion processes that clean the surface and provide a more chemically reactive oxide. Although the result is not as good as that of the common aluminum and titanium treatments,
|
Figure 6 Arrhenius plot of incubation times prior to hydration of FPL aluminum under various conditions. (From Ref. 45.) |
performance is frequently adequate. Improvements to grit blasting, based on either performance or cost, have been reported for individual steels [48-54]; however, rankings of different treatments commonly vary from researcher to researcher because of different steels or exposure/test conditions.
Deposited coatings often provide better bond durability than native surface treatments. For example, optimized conversion coatings can provide a microscopically rough surface that is resistant to corrosion [55-59]. They serve to stabilize the surface from degradation and to form physical bonds with the adhesive/primer. Smaller-grain coatings tend to be preferred over larger-grain coatings as they provide better physical bonding and greater resistance to fracture. Again, differences in the adherend metallurgy can cause differences in the coating morphology and chemistry. Nonetheless, such conversion coatings and other deposited coatings provide the best durability for steel bonds.
Alternative deposited coatings involve thermal spray, such as plasma spray or two — wire arc [60]. Substrate alloy effects are minimal and coating chemistry and morphology can be designed for specific applications. Corrosion protection and good adhesive bond strength can be provided and maintained [61-64].
Joints made with steel and other metals can also be subject to cathodic disbondment if they are immersed in an electrolyte and subjected to a cathodic potential, such as that created when the adherend is in electrical contact with a more electrochemically active metal [65]. Although corrosion of the adherend is suppressed via cathodic protection, the rate of bond failure is increased. After an induction period that depends on the imposed cathodic potential and temperature [65], interphasal debonding occurs [66,67]; such disbondment does not occur in the absence of a cathodic potential. Several mechanisms have been proposed to explain this phenomenon. These include hydrogen evolution at the steel substrate [65], degradation and weakening of the polymer in the high pH environment generated at the interface [66-69], osmotic pressure resulting from lead chlorides formed at the interface by dehydrohalogenation of the polymer [68], and breaking of secondary and primary bonds at the interface [1].
Bond failure can also occur if the surface is anodic relative to another joint component. An example would be clad aluminum adherends where a thin layer of pure aluminum overlays the base alloy. Such a surface layer is designed to be more corrosion resistant than the alloy, but to act as a sacrificial anode should corrosion occur. Although this approach works well for corrosion protection of the substrate material, it can be a disaster for bonded material if the adherend surface/interface corrodes. As a result, American companies tend to use unclad aluminum for bonding and provide other means of corrosion protection, such as painting [1,70]. On the other hand, European companies commonly use clad adherends, but with a thicker oxide (CAA) [6,18,71-73] that provides bondline corrosion protection.
In contrast to aluminum and steel, titanium adherends are stable under conditions of moderately elevated temperatures and humidity. Although moisture has been shown to accelerate the crystallization of the amorphous oxide of titanium adherends anodized in chromic acid (CAA) to anatase [74], the crystallization, along with the resulting morphology change, is very slow relative to the changes observed with aluminum and steel. In the wedge test results of Fig. 1, the adherend surfaces underwent no change in morphology or crystallinity. Failure of the CAA specimens remained within the adhesive, with the physical bonds provided by the microscopically rough oxide remaining intact [74]. Identical results were also observed for wedge tests performed in boiling water, i. e., crack propagation for CAA adherends was entirely within the adhesive [75]. For moderate conditions, the key requirement for a titanium treatment is a convoluted microrough surface to promote physical bonding. Critchlow and Brewis extensively reviewed reported results of different surface treatments for titanium [76].
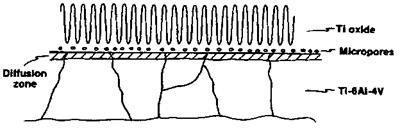
At elevated temperatures where titanium alloys would be the adherend of choice, a different failure mechanism becomes important. Because the solubility of oxygen in titanium increases with temperature, the oxygen in a CAA or other oxide diffuses or dissolves into the metal, leaving voids or microcracks at the metal-oxide interface and embrittles the metal near the interface (Fig. 7). Consequently, stresses are concentrated over small areas at the interface and the joint fails at low stress levels [75,77]. Such phenomena have been observed for adherends exposed to 600° C for as little as 1 hour or 300° C for 710 hours prior to bonding [75] and for bonds using a high-temperature adhesive cured at 371°C [78] or 400°C [75].
To prevent this failure mode, thick oxides, such as those grown by CAA, must be avoided in high-temperature applications. Two types of surface treatments that show some
|
Figure 7 Schematic representation of oxygen diffusing or dissolving from the oxide into the titanium metal at high temperatures. The interphase is weakened with the formation of voids, porosity, and microcracks and with the embrittlement of the interphasal metal region. (From Ref. 75.) |
compatibility with high temperatures have been proposed but both have had problems with consistency under long term testing. Plasma-sprayed metallic titanium coatings were proposed at first and showed promising initial results [75,79]. By properly controlling the deposition parameters, a fractal-like microrough coating is obtained that provides physical bonding with the adhesive. Only a thin native oxide is present and this is apparently insufficient to cause the type of failure described above. Plasma-sprayed adherends have been heated to 450°C for 165 hours prior to bonding and tensile testing and have been bonded with a 400°C-curing adhesive and wedge-tested at 230°C for 1000 hours [18,75]. In both cases, failure occurred within the adhesive, indicating a stable interphase. In later tests, cohesive failure within the coating during wedge tests showed that additional development was needed.
Another titanium treatment that has been evaluated for high-temperature applications is sol-gel coatings [78,80,81] (see below). Specimens retained 50-60% of their initial single lap tensile shear strength after 10,000 hours at 177°C although interfacial failure increased to about 60%. Improvements have been slow because of the long duration tests to distinguish between different variations and the failure of more accelerated tests to give reliable, consistent results. These investigations illustrate the difficulty in designing materials and processes for extreme conditions that cannot be accelerated without introducing new degradation mechanisms.
Another means by which temperature can influence bond durability is through stresses that develop when different parts of a joint have different coefficients of thermal expansion (CTEs). This consideration is especially important when different classes of materials are being bonded together. Typical CTE values for selected materials are given in Table 2 [82]. Polymer CTEs usually are 10-100 times those of other materials. Stresses begin to develop across the interphase once the adhesive cures to a solid (rubbery) state and the joint begins to cool [82]. As long as the adhesive is above the glass transition temperature (Tg), it will generally be compliant enough to relax and accommodate these interphasal stresses. However, once Tg is reached, the adhesive is less compliant and stresses begin to build up. Thus the thermal stresses in a joint will depend on the CTE differences between the substrate(s), adhesive, and any overlying layers or films and on the
|
Table 2 Coefficients of Thermal Expansion
Source: Ref. 82. |
degree of cooling below Tg. One way to minimize these stresses is to blend low and high CTE polymers to match the CTE of the substrate—a procedure most relevant to polymeric substrates [83]. Another is to incorporate mineral fillers into the adhesive to reduce its CTE [82]. In cases of mismatched adherends, e. g., composite to aluminum, a near room-temperature-curing adhesive may be the best solution [84,85].
 9 июля, 2015
9 июля, 2015  Malyar
Malyar 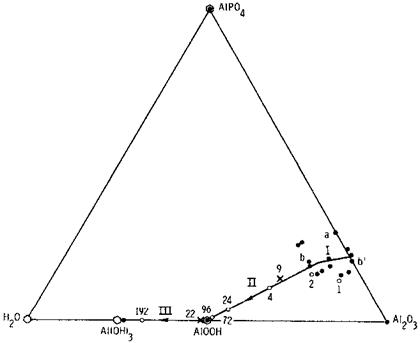
 Опубликовано в рубрике
Опубликовано в рубрике 