Mohamed M. Chehimi and Ammar Azioune
Interfaces, Traitement, Organisation et Dynamique des Systemes (ITODYS), Universite Paris 7- Denis Diderot, Paris, France
Eva Cabet-Deliry
Laboratoire d’Electrochimie Moleculaire, Universite Paris 7 — Denis Diderot, Paris, France
The thermodynamic work of adhesion (W) is by definition the free energy change per unit area required to separate to infinity two surfaces initially in contact with a result of creating two new surfaces (see Fig. 1). It is related to the intermolecular forces that operate at the interface between two materials, for example, an adhesive and an adherend. However, in practice, W may be obscured by other factors (e. g., mechanical interlocking, interdiffusion) since it is always a few orders of magnitude lower than the measured adhesive joint strength [1,2]. One important contribution to practical joint strength is the energy loss due to irreversible deformation processes within the adhesive. Nevertheless, Gent and Schultz [3] showed using peel strength measurements that viscoelastic losses were proportional to the reversible work of adhesion. For this reason, it is important to determine the nature of interfacial chemical and physical forces and to understand how they control the reversible work of adhesion.
In 1964, Fowkes [4] proposed that both the reversible work of adhesion (W) and the surface tension (y) had additive components:
W = Wd + Wp + Wh + Wm + •••
and
y = y d + yp + yh + ym + •••
since the intermolecular attractions at interfaces result from independent phenomena such as dispersion forces (d); dipole interactions (p); and hydrogen bonding (h); a subset of Lewis acid-base interactions, metallic bonds (m), etc. For convenience these intermolecular interactions were split into additive dispersive and nondispersive forces, the latter being unfortunately attributed to polar interactions including the hydrogen bond or acid-base interactions. However, as early as 1960, Pimentel and McClellan demonstrated that the
|
Figure 1 Definition of the work of adhesion, W. |
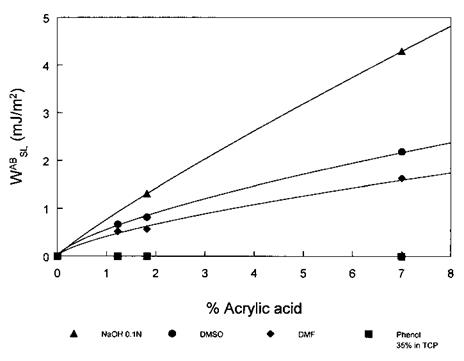
heat of hydrogen bonding between two distinct molecules was related to the acid strength of the proton donor (or electron acceptor) and to the base strength of the proton acceptor (or electron donor) and was completely unrelated to their dipolar moments [5]. This led Fowkes to propose that the so-called ‘‘polar’’ term in the reversible work of adhesion was due to Lewis acid-base interactions (including hydrogen bonding) [6], whereas the true contribution of permanent dipole-dipole interactions and dipole-induced dipole interactions could rather be lumped together with the dispersive interactions term, since it is negligible in the condensed phase (ca. 1%) [7]. Distinguishing between acid-base interactions and ‘‘polar’’ interactions is thus fundamentally important and has also a practical implication since Fowkes demonstrated for complex systems that the former but not the latter led to a substantial improvement in adhesion. It is also important to point out that acid-acid and base-base interactions do not improve adhesion for they are of the van der Waals type only [8-10]. This is illustrated by the determination of the acid-base contribution to the work of adhesion (wSL8) of liquids to poly(ethylene-co-acrylic acid) (P(E-AA)) of varying percentage of acrylic acid [8]. Figure 2 shows for dimethylformamide (DMF), dimethyl sulfoxide (DMSO), and 0.1 N NaOH solution (all three test liquids are basic) the Wgj8 increases with the percentage of acrylic acid. By contrast, W^8 for the phenol solution (Lewis acid) in tricresyl phosphate is zero and independent of the acrylic acid content in the copolymer.
Another important example of the role of acid-base interactions concerns polymer adsorption: Fowkes and Mostafa [11] demonstrated that the amount of poly(methyl methacrylate) (PMMA) (electron donor or Lewis base) adsorbed onto silica (electron acceptor or Lewis acid) was much higher than that of adsorbed chlorinated poly(vinyl chloride) (CPVC) (Lewis acid). When CaCO3 (Lewis base) was used as the substrate, CPVC adsorbed with a greater amount than PMMA. In the case of the PMMA-silica system, it was demonstrated that the acid-base properties of the solvent were of significant importance since the solvent can interact via specific acid-base forces with the polymer (chloroform-PMMA interaction), or can preferentially adsorb onto the substrate (tetra — hydrofuran-silica interaction). 8oth phenomena result in hindering polymer adsorption. By contrast, in a noncompeting solvent such as CCl4, a much higher adsorbed amount of PMMA was obtained onto silica because in this case the polymer-substrate acid-base interactions were maximized.
|
Figure 2 Acid-base contribution to the work of adhesion (WSL8) determined by contact angle measurements for various liquid-acrylic acid copolymer pairs versus the acrylic acid content. |
The pioneering developments of Professor Frederick M. Fowkes regarding the acid-base theory in adhesion have attracted the attention of several laboratories. A Festschrift in his honor on the occasion of his 75th birthday was published in 1991 [12]. This monograph constitutes an important step in the history of acid-base chemistry in general and adhesion science in particular. In the 1990s, progress in science and technology accomplished by academic and industrial researchers confirmed that acid — base interactions were a key parameter in improving adhesion, adsorption, dispersibility, solubility, and mixing of polymers and other materials [12-19]. These specific interactions even became measurable using scanning probe microscopy [19-21] (see Section IV. D). However, discordance of opinion or discrepancy also appeared on both the repulsive aspects of acid-base interactions, and the reliability of the van Oss-Chaudhury-Good (vOCG) theory [22] to calculate acceptable values of the acid-base components of the surface free energy. There was thus a need for a second ‘‘testament’’ on acid-base interactions in adhesion science and technology, which has recently been edited by K. L. Mittal [19].
The aim of the present contribution is to review the role of acid-base interactions in adsorption, wetting, and adhesion, and the methodologies and techniques to characterize the acid-base properties of materials. Examples have been selected from the authors’ research work and from a survey of the literature. This chapter is organized into the following three sections: definition, properties, and strength of acid-base interactions; theory of acid-base interactions in adhesion; and experimental assessment of acid-base properties of polymers and other materials.
 22 июня, 2015
22 июня, 2015  Malyar
Malyar 
 Опубликовано в рубрике
Опубликовано в рубрике 