Xylene blue belongs to the group of so-called patent blues which are sulfonated triphenylmethane dyes that are stable to alkali. The dyes are all characterized by having a sulfo group in the position ortho to the central carbon atom as in the following general formula:
![]() s
s
![]() Na
Na
Sandmeyer first explained the relation between structure and alkali stability, and his erioglaucine was the first dye prepared to take advantage of this relationship.
Erioglaucine A
from ethylbenzylanilinesulfonic acid
and o-sulfobenzaldehyde
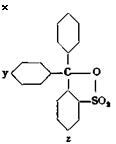 |
Apparently an inner anhydride is formed between the carbinol hydroxyl and the sulfo group, and this causes the stability toward soda and caustic alkali. This assumption appears probable because dyes of the type below are entirely insoluble:
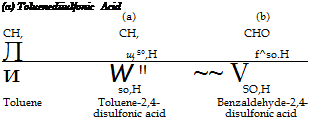
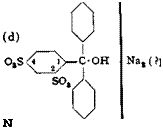 ‘ (c)
‘ (c)
—SOjH/ —CH[C6H4′ N(C1HS)1], —►
~!SO, H
Leuco xylene blue VS Xylene blue VS
To 46 grams (0.5 mole) of pure, boiling toluene is added, over a period of 15 minutes, 80 grams of 100 per cent sulfuric acid, and the mixture is heated at 125°C. for 1 hour. During this time the toluene disappears completely. The mixture is cooled to 30°, and 220 grams of 66 per cent oleum is added with thorough stirring over a period of 30 minutes. The solution is then heated for 4 hours at 125°, converting all of the toluene to the disulfonic acid. The mixture is diluted with 400
grams of sulfuric acid (66° Be) and transferred to a porcelain beaker set up with an efficient iron stirrer.
 5 декабря, 2015
5 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 