|
OH OH OH
SO, H NO* Phenol Disulfonic acid Trinitrophenol |
In a glass’, iron, or porcelain sulfonation vessel, 94 grams of phenol of the highest quality is heated to 100°C., and 300 grams of 100 per cent sulfuric acid is added with stirring at such a rate that the temperature remains below 110°. The mixture is heated for an hour at 100-110° to complete the sulfonation of the phenol, converting most of it to the disulfonic acid. The mixture is then cooled to 0° in an ice-salt bath, and
3.5 moles of nitric acid, in the form of 50 per cent mixed acid, is added over a period of about 3 hours.
|
Fig. 26. Stone filter funnel for strongly acid precipitates: (1) vacuum connection; (2) discharge line for removing filtrate; (3) air outlet; (4) connection to wash water, or to the sewer for emptying. |
Mixed acid is prepared industrially from very concentrated nitric acid and the most highly concentrated oleum. Ordinarily, nitric acid of sp. gr. 1.48 is mixed in large iron kettles with 40 per cent oleum with cooling by water. This procedure should not be used in the laboratory because of its danger. For laboratory purposes, nitric acid of sp. gr. 1.50 to 1.52 is mixed with an equal weight of 100 per cent sulfuric acid.
After all of the nitric acid has been added, die mixture is allowed to stand overnight at room temperature, then it is warmed very slowly, with stirring, on a water bath at 30° for 1 hour. The temperature is then raised to 45°, but no higher or the mixture may suddenly heat up spontaneously. If this happens, even though an explosion may not occur, the mixture spews out of the kettle. The reaction cannot be completed at 45°, however, so a small portion of the reaction mixture (about 55 cc.) is transferred to a 1.5-liter porcelain beaker and heated on a sand bath to 110-125° with stirring. The rest of the mixture is added dropwise with constant stirring to die preheated portion at such a rate that it
|
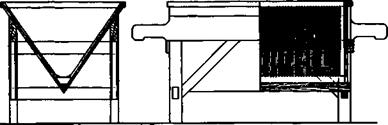
Fig. 27. Portable filter for coarsely crystalline precipitates. |
does not foam over. The mixture is heated another 30 minutes at 110-120°, and then sufficient water is added slowly, still at 120°, to make the mixture 40 per cent sulfuric acid (about 700 cc. water is required). Some nitric oxide is generated, but only a small amount if the nitration has been carried out properly. On cooling, the picric acid precipitates quantitatively, since it is insoluble in 40 per cent sulfuric acid. The mother liquor contains some tar and other decomposition products. The product is separated by filtering the cold solution through a cotton filter and washing with cold water. Chemically pure picric acid is obtained in a yield of about 210 grams.
Similar methods are used for preparing Martius yellow (2,4-dinitro-
1- naphthol) and naphthol yellow S (l,2-dinitro-4-naphthol-7-sulfonic acid), as well as dinitrocresol and other polynitro compounds. If the concentration of the mixed acid is sufficiently high, the reaction succeeds with nearly the theoretical amount of nitric acid.
Technical Observations. All strongly acid liquids, such as that described above, must be filtered through a material which is resistant to acid. Stoneware funnels (Fig. 26) or filter cloths of nitrocotton (nitro filters) are used in practice. The nitrous fumes generated during the nitration are condensed to form nitric acid in towers filled with Raschig rings.
 7 октября, 2015
7 октября, 2015  Pokraskin
Pokraskin 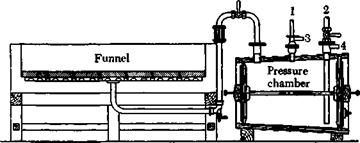
 Опубликовано в рубрике
Опубликовано в рубрике 