The rfaphthalenesulfonic acids find very little direct use in the preparation of dyes, but they are of great technical importance as steps in the preparation of important intermediates such as /З-naphthol and ft- naphthylamine, dihydroxynaphthalenes and aminonaphthols, and numerous naphthylamine-, naphthol-, and aminonaphthol-sulfonic acids.
As was pointed out in the section on orientation rules, the position in the naphthalene nucleus taken by an entering sulfo group depends on the sulfonation temperature. The a position is favored at low temperatures, the ft position at higher temperatures. Accordingly, sulfonation to produce the a-sulfonic acid is carried out at as low a temperature as possible, at least below the melting point of naphthalene, and the naphthalene must be used in a finely pulverized form (see also the preparation of 1,5- and 1,8-naphthylaminesulfonic acids, page 214). The preparation of the ^-sulfonic acid, on the other hand, is carried 6ut at the highest temperature possible without causing decomposition, about 160-170°C. Even under these conditions, some of the a-isomer is always formed, the amount being at least 15 per cent according to the results of various experimenters. The complications involved in this sulfonation have been cleared up largely by the work of O. N. Witt.[40]
The procedure to be followed usually varies, depending on whether the naphthalene-0-sulfonic acid is to be isolated as such or nitrated or sulfonated without actual isolation. In the latter case, since an excess of sulfuric acid must be used anyway, the procedure of Witt60 is advantageous and is used widely in industry. In this process, an excess of sulfuric acid is used in the sulfonation for the purpose of converting the a-sulfonic acid into the disulfonic acid as completely as possible, since the а-compound is further sulfonated much more easily than the ^-sulfonic acid. On the other hand, when the /З-sulfonic acid must be isolated as such, cost considerations demand that the minimum amount of sulfuric acid be used (see 0-naphthol, page 187).
|
1,6- and 1,7-Naphthylaminesulfonic Acids (Cleve Acids)
|
The 1,6- and 1,7-naphthylaminesulfonic acids have long been very important as components for azo dyes, particularly as the middle component in polyazo dyes. They are used in the preparation of the important black cotton dyes of the type of Columbia black FF, and also of a whole series of developed dyes, such as the important naphthogene blue, Zambesi black V, and others. Sulfonic acids of this type are also frequently used in preparing dyes of the Sirius blue series (Bayer).
The sulfonation is best accomplished according to Witt’s procedure, as described in the following paragraphs.
In a sulfonation and nitration vessel as described on page 101 *( Fig. 19), 128 grams of naphthalene of the highest quality is heated over a free flame to 165°C. with continuous stirring. This temperature is maintained while 206 grams of 94 per cent sulfuric acid (66° Вё) is run in slowly. The addition should be made over a period of at least 30 minutes, or too much of the a acid is formed and the yield is lowered. The mixture is then heated for 30 minutes at 165° in order to convert as much as possible of the a acid into the disulfonic acid so that the final product contains the 1,6 and 1,7 acids, practically free from isomeric a-sulfonic acids. The mixture is allowed to cool to 60° while being stirred, but external cooling is not applied since this causes the formation of a precipitate adhering to the walls of the vessel. If such a precipitate does form, it must be scraped from the walls, and if necessary, from the stirrer, and broken up. When the temperature has dropped to 60°, the reaction mixture is diluted with 300 grams of 90 per cent sulfuric acid. (In industrial processes, the monosulfonic acid at this stage is forced by air pressure over into the nitrating vessel. The diluting acid is added first, and it is important that the mixture is not cooled too much since separation of the Д-sulfonic acid may occur under some conditions causing the mixture to solidify and clog the tube.) The mixture is now cooled further with continuous stirring, and when the temperature has dropped to 25°, very slow, dropwise addition of 103 grams (1.0 mole)
of 62 per cent nitric acid (40° Be) is started. Since the nitro compound formed is much more soluble in sulfuric acid than is the naphthalene- sulfonic acid, there is no longer any danger of the reaction mixture solidifying after the nitration has been started. Therefore, the reaction mixture is cooled in ice to 10° after a few grams of nitric acid has been added, and the nitration is completed at this temperature. When about half of the nitric acid has been added, the mixture is examined to see that no deposit has formed on the walls of the vessel and no large lumps are present. If present, these aggregates must be broken up since solid crusts or thick lumps of crystallized naphthalenesulfonic acid will resist nitration even on very long standing. Similar precautions must be taken in processes which use less sulfuric acid for reasons of economy and in which, therefore, the danger of solidification is still greater. When all of the nitric acid has been added (about 2.5 hours), the mixture is allowed to stand for at least 12 hours, after which a nitrometer test should show the presence of not more than about 2 per cent of the nitric acid used. The viscous, but clear, brownish solution is poured into 2 liters water. Practically no nitrous acid should be generated.
The resulting acid solution is heated to 75° and a 20 per cent solution of ferrous sulfate is added until no more nitric oxide is formed. A stream of air is then blown through the liquid until a drop of the solution, greatly diluted with water, fails to give an immediate blue coloration on starch — iodide paper. It is essential that these conditions be met, otherwise side reactions occur in the subsequent reduction.
The ferric salts formed from the ferrous sulfate also give a reaction with starch — iodide paper, but the coloration appears only after one or two seconds, and then it begins to form around the edge of the test drop. An experienced person will not mistake the two reactions; it is safer, however, for the beginner to use, instead of starch-iodide paper, the so-called “sulfone reagent” (4,4-diaminodiplienylinetliane — 2,2’-sulfone) which does not react with ferric salts and which is not destroyed by the strong acid. For further details, see page 243, in the section on tlie diazotization of aniline.
The reaction mixture is now treated with 50 grams of magnesium carbonate, and then with enough finely powdered chalk (about 320 grams) to neutralize all of the free sulfuric acid. (Slaked lime can also be used, but care must be taken not to make the mixture strongly alkaline because free alkali or alkaline earth hydroxide decomposes the nitrosulfonic acids.) The thick paste of calcium sulfate is filtered on a large suction funnel, and the residue is pressed out thoroughly and washed repeatedly with hot water until the washings are only slightly yellow in color. Complete washing out is not to be recommended because the volume of wash water required is too large; the total volume of filtrate and washings should be about 2.8 liters.
Reduction. The solution of the nitrosulfonic acids is made slightly but distinctly acid to Congo red by the addition of hydrochloric acid. In a 2.5-liter reduction beaker, preferably of copper, are placed 250 grams of finely powdered gray cast iron (see pages 75 and 77) and 250 cc. water. The mixture is heated to boiling and the iron is etched by the addition of 10 cc. glacial acetic acid. After boiling for a short time, 20 grams of crystalline sodium acetate is added and then the weakly acid solution of the nitrosulfonic acids is added, over a period of 1 hour, while the mixture is boiled and stirred vigorously (the iron must be churned up!). A considerable part of the solution evaporates but the total volume should not decrease to less than 1 liter. When the addition is completed, boiling is continued for 20 minutes, and then a test is made to see whether a drop of the solution is nearly colorless on filter paper. It will not be completely colorless, but it should, in no case, be strongly brown or yellow. The boiling solution is neutralized by the addition of calcined magnesia (about 20 grams) until a test drop on filter paper shows the presence of an easily filterable iron oxide precipitate and the solution is weakly? but distinctly, alkaline to litmus. The solution should also be tested on filter paper with sodium or ammonium sulfide to determine whether all of the iron has been precipitated. If this is not the case, the residual iron is precipitated with a small amount of ammonium sulfide. It is absolutely necessary to remove the last traces of iron to avoid rapid oxidation of the Cleve acids in subsequent steps.
The solution of the magnesium salts of Cleve acids is filtered with suction and the solution evaporated, if necessary, to 1 liter, using a porcelain dish over a free flame and a wooden ■propeller over the dish.
The concentrated solution is made strongly acid to Congo red by the addition of about 100 cc. concentrated hydrochloric acid and left to crystallize for a day while being stirred continuously. Both of the Cleve acids (1,6- and 1,7-naphthylaminesulfonic acids) separate slowly, although they are rather insoluble in water once they have precipitated. After 24 hours, the precipitate is filtered off and washed thoroughly with a large volume of water.
To separate the crude acids, the precipitate is dissolved in 800 cc. hot water and enough ammonia (or soda solution) is added to make the solution distinctly alkaline. Sodium chloride is now added in sufficient amount to make the solution 10 per cent with respect to salt. The difficultly soluble salt of the 1,7 acid separates in the course of a day in the form of greasy, lustrous, yellowish plates which are filtered off and washed with 10 per cent salt solution and then with a small
amount of cold water. Hydrochloric acid is added to the filtrate to make it distinctly acid to Congo red, and the solution is allowed to stand with occasional stirring for 2 days. The resulting precipitate of the
1.6 Cleve acid is filtered off, washed with water, and dried at 100°.
The yield of 1,6 Cleve acid is about 80 grams (mol. wt. 223), that of
1.7 Cleve acid about 75 grams of the sodium salt (mol. wt. 245).
The mother liquor contains appreciable quantities of the impure products which are discarded or, in large scale operations, recovered by evaporation.
Technical Observations. The pure 1,6- and 1,7-naphthylaminesulfonic acids give practically identical dyes. Frequently it is advantageous to use a mixture of the two acids because the resulting dyes are stronger, particularly in the case of black polyazo dyes (e. g., Columbia black or Zambesi black V).
The 1,7 Cleve acid is usually obtained in higher purity than is the 1,6 acid, so the 1,7 acid is generally used in preparing complex dyes. The presence of 1,8- naphthylaminesulfonic acid in the 1,7 Cleve acid is easily recognized by diazotiza — tion, and heating the diazonium compound. The 1,8 compound yields naphthsultone, which forms an insoluble precipitate which can be filtered off and weighed (see page 217).
Naphthalene-/?-suIfonic Acid and y3-Naphthol
SO. H
85% 15%
In the preparation of naphthalene-/J-sulfonic acid, the sulfuric acid must be used up very completely since /J-naphthol is so cheap that only the cheapest process for its preparation will survive.
In the apparatus described on page 101, 256 grams (2 moles) of naphthalene is heated to 165°C. over a free flame with continuous stirring, and over a period of 30 minutes 280 grams of 94 per cent sulfuric acid (66° Вё) is added, while the temperature is held between 163 and 168° by careful regulation of the flame. The dropping funnel is now removed and in its place is installed a bent glass tube fitted tightly into the cover (with a cork or asbestos paper). During the course of the sulfonation, water and naphthalene distill out through this tube. The mixture of naphthalene and sulfuric acid is heated with continued stirring for an hour at 165°, then an hour at 167°, another hour at 170°, and finally an hour at 173°. During this time, about 30 grams of water and 25 grams of naphthalene are collected in the receiver. An appreciable amount of naphthalene deposits on the cover of the reaction flask, but this is neglected. The flame is now removed and the apparatus dismantled. The resulting mixture contains, in addition to naphtha-
lenesulfonic acid, a certain amount of sulfone, free sulfuric acid, and some disulfonic acid, along with tars. It should be almost colorless. The mixture is poured, still hot, into 1.8 liters water.
The resulting solution of the free sulfonic acid is partly neutralized by the careful addition, with good stirring, of 60 grams of soda ash. Then, 360 grams of salt is added slowly. The solution soon solidifies in large lumps which make further stirring difficult. Nevertheless, stirring must be continued until the mixture appears completely homogeneous, for only in this way can an easily filterable precipitate be obtained and complete solution of the salt be achieved. The duration of stirring depends on its speed, but should be at least 6 hours to ensure complete separation. The precipitate is then filtered off on a large suction funnel with a cotton filter, sucked as dry as possible, then transferred to a moistened, strong, cotton cloth and pressed out in a screw press, carefully at first and then as strongly as possible. The pressing should be carried on for at least 2 hours, otherwise too much mother liquor remains in the precipitate. The resulting hard mass is broken up and dried completely at 100-120°.
The yield of “/? salt” is about 165 per cent calculated on the basis of naphthalene, or about 400 to 420 grams. From the mother liquor, containing some of the a acid in addition to tar and a trace of /? acid, Glauber salt may be recovered.
Alkali fusion of sodium naphthalenesulfonate is one of the most important organic-technical operations. With the low price of naphthol, it is not surprising that only a few manufacturers undertake the preparation. Very cheap materials, such as coal, soda, and sulfuric acid, are essential.
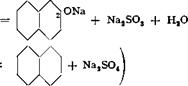 /’V^SO. Na
/’V^SO. Na
2| 8 + zNaOH
side reaction
The sodium sulfonate must be pulverized very finely in order to get good fusion with the alkali. In the laboratory, this is accomplished most easily by grinding the salt in a coffee grinder.
The fusion apparatus described on page 87 (Fig. 14) is placed directly over a small Fletcher burner and charged with 200 grams of solid, chlorate-free sodium hydroxide in large sticks, and 60 cc. water. (Fusions using chlorate-containing alkali give lower yields and are,
moreover, very dangerous. Explosive!) The alkali is melted with a large flame. The melt is water clear, and foams as the temperature is raised gradually to 270°C., at which point the foaming ceases. The powdered sodium sulfonate is now added, with continued stirring, in teaspoon portions, and the temperature is raised slowly to 290°. The dry sodium salt is seen to disappear gradually as the dark colored, lustrous
 17 октября, 2015
17 октября, 2015  Pokraskin
Pokraskin 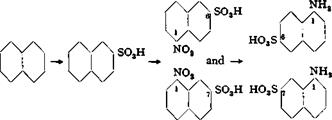
 Опубликовано в рубрике
Опубликовано в рубрике 