K. VACUUM DISTILLATION IN THE LABORATORY AND PLANT
Distillation under reduced pressure, or vacuum distillation, is one of the most important techniques in the dye field. Certain products are distilled under reduced pressure because they decompose at their boiling points under normal atmospheric pressure, while in other cases, vacuum distillation has other advantages. Thus, the heat lost by radiation is less at the lower temperatures required, and furthermore, heating can frequently be done with steam so that the apparatus can be used anywhere in the plant without danger of fire. One other very important factor, which in itself is a sufficient reason for working with many substances under reduced pressure, is the easier separation of mixtures made up of compounds whose boiling points lie close together. Thus, the three isomeric nitrotoluenes can be separated satisfactorily only by distillation in vacuum, and the alkylbenzylanilines can be obtained pure only by vacuum distillation.
The laboratory apparatus used for many years for fractional distillation was poorly designed in that it did not give proper reflux ratios. Too much emphasis was placed on the form, of the column and no attention was given to the importance of the reflux which is necessary to give a systematic washing out of the higher boiling constituents in the column. The reflux is regulated by means of a condenser above the column, arranged so that any desired amount of distillate can be condensed and returned through the column.
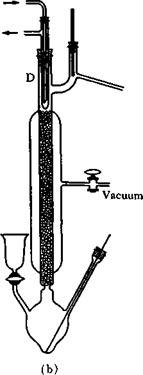
Figure 37a and b shows a practical glass column which is very valuable for laboratory work. The column packing consists of small glass Raschig rings in random orientation, giving excellent distribution of the returning liquid.
The partial condenser (D) is cooled by air, water, alcohol, etc., depending on the desired efficiency. For example, the condenser can be
maintained at exactly 100°C. by dripping distilled water continuously into the condenser jacket, or in larger installations, a standard condenser can be used and the cooling medium kept at its boiling point.
 |
 |
It is very important that a uniform rate of return of the distillate be maintained. Hence, it is necessary that the whole column be well
Fig. 37. Fractionating column with partial condenser (D) (dephlegmator).
insulated and protected from air currents, for example, by means of a vacuum jacket (Fig. 37b). The source of heat (for example, an oil bath) must also be kept constant. A distillation can be disturbed merely by a person passing by. It is preferable, therefore, to carry out such operations in a separate laboratory and not in the general workroom.
In addition, the vacuum must be held absolutely constant. Hence, it is necessary, when a water pump is used, that the water pressure be
great enough so that the pump does not “suck back.” If the water pressure is not high enough, it may help to attach to the pump a 10-meter down-tube of sufficient width. Such a tube acts as a barometric tube and makes possible a very constant vacuum equal to the vapor pressure of the water.
|
Fig. 38. (a, b) Kubierschky columns and (c) Raschig column. Diameter of columns is 50-120 cm.; height, 8-16 m. The upper portion (1-2 m.) is cooled externally in rectification operations; the rest of the column (7—15 m.) is well insulated and the top opening is closed. |
An iron apparatus can be constructed for distilling larger quantities. In this laboratory, a 2.5-meter column with a 4-liter distilling flask has been in use for many years.
Separation of isomeric nitrotoluenes, chloronitrobenzenes, etc. is easily accomplished with columns of this type. Some mixtures, whose constituents have boiling points very close to each other, can be fractionated provided that the substances crystallize so that the pure compounds can be separated from the eutectic mixture by centrifuging. An example is the mixture of nitrochlorobenzenes, described on page 90.
On the other hand, a mixture of o — and p-chlorotoluene, for example, can not be separated. A test must be made with fairly large amounts of material to learn whether any particular mixture can be separate.
Industrial fractionating columns are constructed in a similar way, and special designs have been evolved to achieve efficient contact between vapor and reflux. Some of these columns are shown in Figure 38. In large equipment, heat is supplied by superheated steam, or oil, or by direct firing in cases where good fractionation is not necessary, such as in the distillation of aniline, naphthol, or diphenylamine. Figure 40 shows a very simple vacuum distillation arrangement which works very well despite its apparent primitiveness. Frequently, it is important that a trap be installed between the vacuum pump and the receiver to prevent any sublimate from getting into the pump. Many manufacturers supply equipment of this kind.
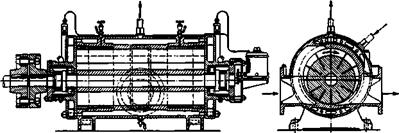
The vacuum is produced by reciprocating pumps which reduce the pressure to about 50 mm. of mercury. Lower pressures, down to about 8 mm., can be obtained by using two pumps in series, but this practice is undergoing change. In recent years, rotatory pumps have been finding increasing use. One type of these pumps is shown in Figure 39 which is drawn schematically to show the mode of action. The moving slide bars enclose a certain volume of air and drive it toward the exhaust opening, compressing it in the process. The machine can be used either as a compressor or as a vacuum pump to produce about 4 atmospheres of pressure or a vacuum of about 12 mm. As shown in the illustration, the apparatus must be equipped for cooling. The pump is coupled directly to a multiphase motor operating at 1500 to 2000 r. p.m., and a minimal loss of power in transmission is entailed. Frequently, two rotatory pumps are used in series.
Vessels of very large dimensions are often used for vacuum distillation of liquid materials. Aniline, for example, is distilled in quantities of 20,000 kilograms or more in vessels equipped with steam coils. ^-Naphthol and like substances must be handled in smaller quantities, but even with these, 200 kilograms or more are distilled at one time using a different kind of apparatus. The high distillation temperature of /3- naphthol does not permit the use of steam heating, although promising results have been obtained with the Frederking apparatus. Generally, heating must be done by flame, gas firing being preferred because it can be regulated easily. Frequently, such distillations are carried out without the use of an oil bath, but this entails a risk of charring the residual pitch, making it worthless and hard to remove from the vessel. (Naphthol pitch is an important commercial product. It is a glassy, black, brittle mass which is used as an insulation material in electrical sockets.) In large scale vacuum distillations, it is unnecessary to introduce air since no bumping occurs. The whole apparatus must be well insulated and all tubes, which might become clogged, must be easily accessible and arranged so that they can be heated. The receiver is jacketed for either heating or cooling. When the distillation is completed,
|
Fig. 39. Rotary compressor and vacuum pump. |
the liquid distillate is forced out of the receiver in a closed system so that the fumes are jiot bothersome. A large trap is installed between the receiver and the pump to catch water and sublimed material. Especially in the distillation of /З-naphthol, large amounts of fine snow go over and this could foul the pump.
The larger apparatus have several thermometers, one of which extends to the bottom of the distillation kettle to give the temperature of the crude mixture and to indicate the beginning and end of the distillation. When the temperature difference between the vapors going over and the residue in the kettle is about 50°, the distillation should be stopped to avoid charring the pitch.
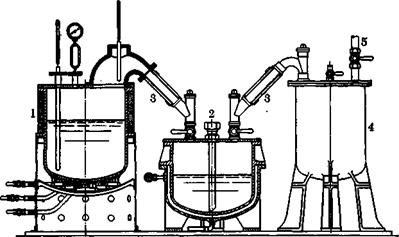
Figure 40 shows an apparatus for the distillation of /З-naphthol. It is heated by gas (three ring burners) and is designed for about 1000 kilograms. A distillation requires about 4 hours. The residual pitch amounts to about 5 per cent of the crude naphthol. (Most distillation apparatus for naphthol have stirring mechanisms so that no charring occurs at the bottom of the kettles.) The distilled naphthol is allowed to solidify in lumps and is centrifuged, after the product has been pulverized.
For distilling liquids, coil condensers are used, or, in some cases, straight-tube condensers with multiple tubes (20 to 30). These condensers can also be heated, so that diphenylamine or other easily melted products can be handled in them in the event that it is not feasible to use steam distillation (see pages 140-142).
A window is often installed in the downward portion of the condenser to permit observation of the stream of liquid.
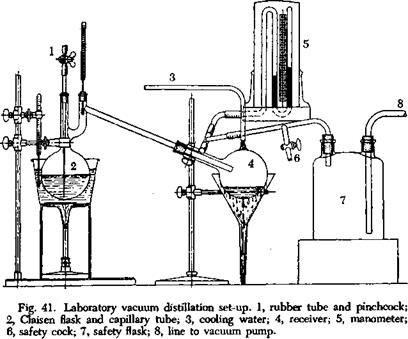
The arrangement sketched in Figure 41 is very satisfactory for laboratory distillations. The distillation flask has two necks carrying, respectively, a fine capillary and a thermometer. The double neck arrangement also prevents spray from being carried over. Usually the flask is heated, not directly, but in an oil bath which is at a temperature 30 to 40°
|
Fie. 40. Vacuum distillation equipment for substances which solidify easily (napnthols, phenylenediamine, etc.). The apparatus for large quantities (1000- 3000 kg.) is equipped with a stirring mechanism to prevent charring. 1, distillation vessel; 2, receiver with steam or water jacket; 3, steam heated tubes to prevent solidification; 4, trap to collect water and sublimate; 5, line to pump. |
higher than the distillation temperature. The capillary is drawn out from an ordinary thin-walled tube. It should be as flexible as a silk fiber and reach to the bottom of the flask. The upper end is closed by means of a rubber tube and screw clamp. Enough air is introduced during the distillation to make it run smoothly.
An ordinary distillation flask with a long neck is used as a receiver. If various fractions are to be collected, the distillation is interrupted after each fraction, and the receiver is changed. This operation takes only a short time. The manometer is not put in the line with the pump, but is attached through a special tube so that no liquid can get into it. It is important to have a safety cock in the system so that a small amount of air can be admitted, and the distillation immediately brought under control if the liquid in the distilling flask begins to boil over. The pump should be separated from the distillation flask by a large safety flask. It is recommended also that the pump be installed directly onto the main line so that it will not be affected by fluctuations in water pressure.
In many cases, a so-called “sword” or “sausage” flask can be used instead of the arrangement described. This is especially to be recommended for the beginner.
|
|
Carrying Out the Distillation. The pump is started and the oil bath is heated to the correct temperature. At first, water or solvent comes over and the pressure must be regulated by means of the safety cock so it is not too low. When boiling ceases, the capillary is adjusted to give a fairly rapid stream of very fine bubbles, and after a time, distillation starts. With liquids, no difficulties are encountered, but solid substances, such as /ї-naphthol, naphthylamine, etc., may clog the exit tube. In this case, the neck of the distillation flask should be heated before distillation begins so that the first drops are superheated. Under some conditions, the side arm must be heated down to the cork in order to get the distillate through before it solidifies. Although there is no danger of cracking the flask if it is made of good glass, it is always advisable to wear goggles. The distillation should take place rapidly. For example, 200 grams of /J-naphthol is distilled in 15 to 20 minutes without trouble in cooling the receiver. The manometer is shut off from the apparatus and only connected from time to time to check the vacuum. Beginners often try to make their apparatus tight by smearing the joints with parraffin, collodion, or other material, but this is a highly undesirable practice. It is much simpler to impregnate good corks beforehand with hot, hard paraffin and then additional coating is entirely unnecessary. Rubber stoppers are used only for high vacuum distillations, but better results are obtained by using apparatus with sealed glass joints, as in the technical distillation of guaiacol, where a mercury pump is used.
The distilled material is melted by heating over a free flame and then poured out into a small porcelain dish. The solidified material is pure and is not recrystallized.
 26 декабря, 2015
26 декабря, 2015  Pokraskin
Pokraskin 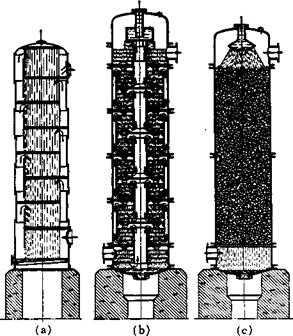
 Опубликовано в рубрике
Опубликовано в рубрике 