Chloramine Yellow FF (Naphthamine Yellow NN) and Thiazole Yellow
NH,
![]()
![]()
![]()
![]()
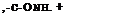
![]() 0"
0"
CH,
 |
p-Toluidine
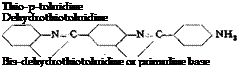
In general, the reaction of sulfur with an aromatic amine gives a substitution product in which the aromatic nuclei of two molecules are connected through a sulfur atom. Such reactions, however, always give mixtures of products, and it is usually impossible to obtain an individual reaction product. Thus, the reaction between sulfur and p-toluidine leads to three products which are easily recognized: thiotoluidine, de-
hydrothiotoluidine, and bisdehydrothiotoluidine. The structures of these are given above.
|
Fig. 36. Iron fusion kettle with copper oil bath for use in primuline, indigo, and alkali fusions, etc. Capacity, about 450 cc. |
A mixture of 214 grams (2 moles) of p-toluidine, 140 grams of powdered sulfur (not flowers of sulfur!), and 2 grams of soda ash is heated to 180°C. in the kettle, equipped with stirrer and reflux condenser, shown in Figure 36a and b. (The soda is added to neutralize traces of acid always present in sulfur. If the soda is omitted, the primuline melt is always dark colored or black.) Hydrogen sulfide is evolved and collected either in sodium hydroxide solution or in towers filled with moistened sodium hydroxide sticks. After about 8 hours when the evolution of hydrogen sulfide abates, the temperature is raised slowly to 220° and held at this point for 5 hours. Hydrogen sulfide evolution now practically ceases, and the melt is ladled out onto a flat rimmed plate where it solidifies to a light yellow, crystalline cake. The yield is 325 grams.
 22 декабря, 2015
22 декабря, 2015  Pokraskin
Pokraskin 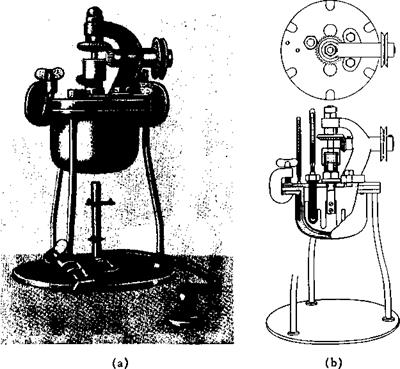
 Опубликовано в рубрике
Опубликовано в рубрике 