Like halogenation and nitration, sulfonation is of the greatest importance in dye manufacture. Most of the water soluble dyes owe their solubility to the presence of sulfonic acid groups. In addition, sulfonic acids are extremely important intermediates in the preparation of phenols, especially those of the naphthalene series.
The majority of sulfonation reactions are effected by treatment with sulfuric acid, either ordinary concentrated sulfuric acid, 100 per cent sulfuric acid, or fuming sulfuric acid (oleum) of various SO3 content The reaction temperature to be maintained varies between wide limits — 0° to about 200° — depending on the reactivity of the starting material. Sulfonation occurs more easily in the naphthalene series than in the benzene series, but more difficultly with anthraquinone. It is greatly facilitated by the presence of phenolic hydroxyl groups and, to a smaller extent, by alkoxy, amino, and acylamino groups. Alkyl groups also have a similar, but quite small, effect. On the other hand, sulfonation is made more difficult by the presence of halogen, carboxyl, carbonyl groups, other sulfo groups, and especially by nitro groups. Dinitro derivatives of hydrocarbons, in general, cannot be sulfonated. In the sulfonation of sulfonic acids, it is frequently advantageous to use the alkali metal salt instead of the free acid; moreover, the addition of Glauber salt often makes the reaction run more smoothly. The temperature at which sulfonation is carried out often influences the position taken by the sulfo group, especially in the naphthalene series (see the section on orientation rules.) In these instances, an already formed sulfonic acid can be more or less completely isomerized, forming a state of equilibrium characteristic of the particular temperature. This behavior has great practical significance in the naphthalene series.
This rearrangement is usually explained by assuming that the sulfo groups are split out at elevated temperatures, with the establishment of an equilibrium such as:
|
SO, H
|
Now, experience has shown that splitting out of sulfo groups from naphthalene compounds, as well as their replacement by hydrogen, by die action of hot aqueous sulfuric acid, and their replacement by hydroxyl in alkali fusions, takes place more easily with the a-sulfonic acid than with the p isomer. Accordingly, the apparent wandering of a sulfo group usually, but not always, occurs from an a to a p position. It should be emphasized, however, that migrations of a sulfo group from one a position to another (e. g., l-napnthylamine-8-sulfonic acid -» 1,4 isomer -» 1,5 isomer), or from one p position to another (e. g., 2,7-naphthalenedisulfonic acid -* 2,6 isomer), have been observed.
In the anthraquinone series, the presence of mercury salts favors sulfonation in the a-position.
Since water is formed in the sulfonation reaction and dilutes the sulfuric acid, an appreciable excess of the add is always necessary in order that its concentration be maintained sufficiendy high throughout the reaction. When sulfonation is done with oleum, usually only the SO3 is used up, so that at the end of the reaction a large excess of sulfuric acid remains. The separation of the sulfonic acid product from the excess sulfuric acid is simplest in the case of sulfonic acid derivatives of amines. These compounds, if they contain an equal number of sulfo and amino groups, are generally so difficuldy soluble in water, and particularly in dilute sulfuric acid, that they are almost completely precipitated on dilution of the sulfonation mixture, and need only to be filtered off and washed.
Polysulfonic acid derivatives of monoamines, on the other hand, behave like the sulfonic acid derivatives of hydrocarbons; usually their acid salts, in which one sulfo group is free and the others are present as alkali metal salts, are salted out quite easily. Monosulfonic acid derivatives of polyamines are, like the free amines, soluble in excess mineral acid (cf. page 33).
Two general methods are employed technically for the isolation of other sulfonic acids. The first method involves neutralizing the diluted sulfonation mixture with Ca (ОН )2 (slaked lime, milk of lime) or with CaCOs (chalk or finely powdered limestone) and filtering off the precipitated CaSQ#. The calcium salt of the sulfonic acid, left in the filtrate, is converted to the sodium salt by the addition of soda or Glauber salt, the precipitated CaCOs or CaSO* is filtered off, and the resulting solution of the sodium sulfonate is evaporated until crystallization occurs, or u necessary, until dry. (Alternatively, the sulfonation mixture may be treated at the outset with the necessary amount of Glauber salt to form the sodium salt, and then lime is added, whereby a solution of the sodium sulfonate is obtained directly after filtering off the CaSC>4.) This process, technically referred to as “liming”, is usable with all sulfonic acids except those that form difficultly soluble calcium salts; it is, however, an inconvenient and rather costly process because it requires a large volume of liquid which must ultimately be evaporated. It is usually preferable, therefore, to use the second general method in which the diluted sulfonation mixture is treated with an inorganic salt (usually NaCl or Na2S04, less often, KC1 or (NH4)2S04) in order to salt out the corresponding salt of the sulfonic acid. This method is not always possible since not all sulfonic acids can be precipitated sufficiently completely. Also it must be remembered that sulfonates isolated
in this way are, as a rule, contaminated with some of the inorganic salt used in the precipitation. This contamination may not interfere with the use of the product, however.
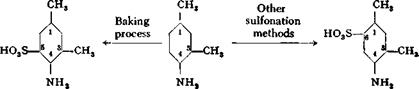
Aromatic amines can often be sulfonated advantageously by a special method applicable only to these compounds. The method involves dry heating of the acid sulfate of the amine (containing 1 mole of H2SO4 for each amino group) to temperatures of about 170-220°, suitably in vacuum. By this so-called “baking process,” the sulfonic acid group is always introduced ortho or para to the amino group, even with polynuclear substances. This method may be used, therefore, to prepare pure sulfonic acids in those cases where other methods lead to mixtures of isomers. Thus, a-naphthylamine gives exclusively l-naphthylamine-4-sulfonic acid by the baking process, whereas other methods give mixtures of the 1,4 and 1,5 isomers. The baking process is also valuable in cases where the desired isomer is not formed at all by other methods, as in the case of m-xylidine. Here, the baking process introduces the sulfo group ortho to the amino group, but other methods put it in the meta position because of the orienting influence of the two methyl groups:
For further details of the baking process, see sulfanilic acid (page 126) and naphthionic acid (page 180).
The unavoidable excess of sulfuric acid in sulfonation reactions using sulfuric acid or oleum causes, under some conditions, such undesirable side reactions as further sulfonation or rearrangement of the sulfonic acid formed initially. These difficulties can be avoided by effecting the sulfonation with the calculated quantity of chlorosulfonic acid in an organic solvent which is not attacked by this reagent (usually nitrobenzene). For further details on this method, see the preparation of 2-naphthol-l-sulfonic acid, page 199.
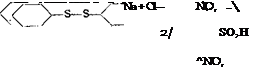
If sulfonation is done with a large excess of chlorosulfonic acid without a solvent, the sulfochloride is formed, as a rule, instead of the free sulfonic acid. For example, large quantities of the mixture of o- and p-toluenesulfonyl chlorides are prepared in this way:
CH,
-SO, Cl
I
SO, Cl
The ortho compound is used in the manufacture of saccharin, while the para compound, which was formerly regarded as a bothersome by-product, has more recently found many uses in dye manufacture and in the pharmaceutical industry.
In sulfonation reactions with chlorosulfonic acid, as well as with sulfuric acid and oleum, small amounts of sulfones are often formed as by-products. The sulfone is formed by the reaction of the sulfonic acid with unchanged starting material under the influence of the dehydrating action of the sulfonation reagent, according to the equation:
Compared with direct sulfonation, the indirect introduction of sulfo groups is of less importance in dye chemistry, although it is used in the preparation of a few technically important compounds. The methods used technically are:
(1) Replacement of an “active” halogen by a sulfo group by treatment with sodium sulfite. The “active” halogen may be aliphatically bound, as in benzyl chloride, or it may be in an aromatic nucleus and activated by negative substituents, such as nitro groups. Examples include the preparation of 2,4-dinitrobenzenesulfonic acid from 2,4-dinitrochlorobenzene (page 103) and the preparation of nitrobenzene-2,5-disulfonic acid from o-nitrochlorobenzene-p-sulfonic acid (page 106).
(2) Action of bisulfite on a nitro compound or on a quinone or quinonemo — noxime, *in which the nitro and nitroso groups are simultaneously reduced to amino groups. Examples are the preparation of l-naphthylamine-2,4-disulfonic acid from a-nitronaphthalene and bisulfite (naphthionic acid as by-product), and the manufacture of l-amino-2-naphthol-4-sulfonic acid from nitroso-0-naphthol and bisulfite (page 201).
(3)
 |
Oxidation of a sulfimc acid, a mercaptan, or a disulfide. For example, the chlorine in o-nitrochlorobenzene is not reactive enough to be replaced directly by the sulfo group according to (1) above; the compound can be converted, however, to o, o’-dinitrodiphenyldisulfide by the action of Ыагвг, and this, in turn, can be oxidized to o-nitrobenzenesulfonic acid.23 The reactions involved are:
![]()
5.
Phenol
The crude paste of sodium benzenesulfonate (about 50 per cent), obtained as described in section 4 above, is used as the starting material. A test is made to determine the weight of solids in the paste, assuming arbitrarily that the solid substance is pure sodium benzenesulfonate. It is theoretically possible to fuse 2.25 parts of sodium benzenesulfonate with 1 part of caustic soda, but practically, only 1.65 parts can be used.
The best material for a fusion apparatus (see Fig. 14a) in the laboratory is copper, which saves on gas because of its good conductivity and which is therefore economical in operation. (It is important that the melt does not come in contact with two different metals, because then a galvanic cell is formed which causes deleterious oxidation and reduction reactions.) The high fusion temperature makes it essential that the stirrer sweep over the whole surface of the fusion vessel (see sketch). The thermometer is inserted in a copper tube closed at the end with hard solder and filled with dry cylinder oil to such a depth that at least 10 cm. of the thermometer is immersed. It may be practical also to insert the thermometer in the hollow shaft of the stirrer (Fig. 14b).
The fusion apparatus is placed directly over a small Fletcher burner and charged with 200 grams of solid (stick) chlorate-free sodium hydroxide and 100 cc. water. (Fusion with hydroxide containing chlorate lowers the yield and, moreover, is very dangerous; explosive/). The hydroxide is melted with a large flame, the melt becoming water-clear and foaming until the temperature reaches 270°C. The melt is heated further to 290° and then is added in small portions to the well stirred melt, the benzenesulfonate paste, corresponding to 330 grams of solid material, which is held at about 100° to keep it liquid (caution, goggles!). The addition, made at such a rate that the temperature inside the vessel remains at 290-300°, requires about 45 minutes.
When the addition has been completed, the temperature is raised to 325° over a period of 30 minutes, and held at this point for 40 minutes while continuous stirring is maintained, after which the melt, while still hot, is poured out onto a shallow plate. After cooling, the mass is broken up and returned to the kettle with 500 cc. water. The
|
Fig. 14. (a) Fusion kettle for alkali fusions, (b) Fusion kettle with central thermometer tube: A, handle, attached by rivets, for securing the kettle; B, opening for introducing gases; C, filling port. |
greater part of the material dissolves easily on careful warming, but a crust of sodium sulfite always remains undissolved. The solution is decanted, and successive additions of water are made until all of the material is dissolved. Not more than 2 liters of water should be required. The combined solutions are heated to boiling in a porcelain dish over an open flame and treated with sufficient 50 per cent sulfuric acid or concentrated hydrochloric acid to make the reaction to thiazole paper almost disappear. The solution is then cooled slightly and filtered through a large suction funnel into a warm flask, and the clear, warm filtrate is treated with stirring with concentrated hydrochloric acid until a permanent blue reaction with Congo red paper is obtained. The solution is then cooled and extracted with three 400-cc. portions of benzene to remove the phenol. The benzene is distilled off and the
phenol is fractionally distilled at ordinary pressure, or better, in vacuum. The yield amounts to as much as 85 per cent of the theoretical quantity, that is, 90 to 100 parts of phenol, melting at about 38°, from 100 parts of benzene.
Technical Observations. The fusion is carried out by direct firing in kettles having capacities up to 2400 liters. The stirrer requires 5 H. P. Fusion of the sulfonate from 1000 kilograms of benzene requires about 6 hours in all, including the mixing operation.
The phenol is not extracted, but is precipitated with sulfuric acid of 40° Be and separated from the salt-containing mother liquor which contains less than 0.4 gram of phenol per liter. The phenol is then steam distilled. Upon concentration of this crude product by use of long rectifying columns, the phenol content— originally about 75 per cent — is brought to about 95 per cent. The remainder of the crude product is water and “salt.” The Raschig column is especially useful for this purpose.
The phenol is then distilled in vacuum. The forerun, as well as the water separated from the crude phenol, contains a considerable amount of phenol and is used in the next fusion run. The residue is also returned to the process so care is taken not to char it. The boiling point of phenol is about 186°C. at 760 mm.
Recording thermometers are frequently used, affording excellent control of the process.
Phenol is also prepared technically by an entirely different method in which chlorobenzene is reacted with sodium hydroxide in the presence of copper at very high temperatures (about 340°) and pressures (about 320 atmospheres). This process is operated on a very large scale by Dow Chemical Company.[15] It has already been mentioned under aniline (page 76) that an analogous reaction between ammonia and chlorobenzene is used in preparing aniline.
 18 сентября, 2015
18 сентября, 2015  Pokraskin
Pokraskin 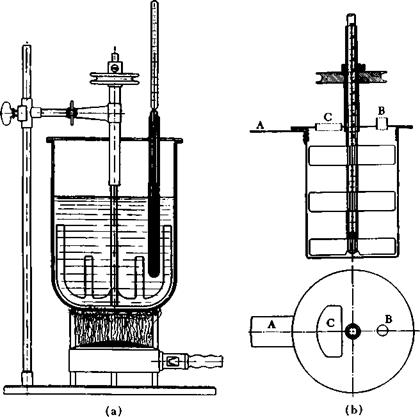
 Опубликовано в рубрике
Опубликовано в рубрике 