In many reactions involved in industrial organic chemistry, continuous, vigorous stirring of the reaction mixture is absolutely essential for good results. This is especially true if some of the reactants are not in solution in the reaction medium, but are only suspended, in either solid or liquid form. Also, when a precipitate is formed during a reaction, stirring is beneficial because it promotes crystallization and prevents the formation of supersaturated solutions which might solidify all at once. Good stirring is necessary even in homogeneous systems if one of the reactants decomposes slowly. Here the unstable compound must be distributed uniformly throughout the whole mass and high local concentrations must be avoided. Stirring is also especially important for strongly exothermic reactions, such as nitrations, where a local excess of the reagent may cause a violent reaction or even an explosion.
Since most organic reactions require a long time, stirring by hand, which often suffices in the analytical laboratory, is usually excluded and mechanical stirring devices must be used. In the laboratory, these mechanical stirrers may be driven by small water turbines, provided that a water pressure of at least two atmospheres is available. An electric motor is used when greater power is needed, as for example, for driving high speed stirrers in reductions, or for stirrers in autoclaves. Sometimes it may be advantageous to have a long power shaft so that several apparatus can be operated simultaneously.
The form of the stirrer itself depends on the nature of the reaction mixture and on the shape and construction of the container in which the stirrer operates.
Naturally, it is most convenient to use open containers, such as beakers, enamded crocks, open kettles, etc., but these cannot be used if an appreciable loss of a substance or a solvent is to be expected. Open vessels are unsuitable also for reactions which generate vapors which must be absorbed or led to the drain because of their poisonous nature, inflammability, or bad odor. In this connection, it should be noted that many of the intermediates used in the organic industry are strongly poisonous and may be absorbed into the body not only through the stomach, but also through the skin, and, as dust or vapor, through the lungs. Examples of some of these compounds are nitrobenzene, aniline, dinitrobenzene, dinitrochlorobenzene, nitroaniline, and phenol Of course, open containers cannot be used in cases where air must be excluded because of the deleterious action of oxygen or of the moisture or carbon dioxide in the air.
When an open vessel cannot be used for any of the above reasons,
recourse is had to a covered kettle or round-bottomed flask — often with three or five necks — which is provided with a reflux condenser or an air condenser, or is connected with an absorption flask to absorb poisonous or irritating vapors. An efficient hood will usually suffice to remove small amounts of gases generated. Except when work is to be done under pressure, a “closed” apparatus must be provided with an opening for pressure equalization. This opening, if necessary, can be protected with a calcium chloride or soda lime tube to remove moisture or carbon dioxide, but it is often sufficient to restrict the entrance of air by means of a capillary tube or a plug of glass wool.
Regarding container materials, it is to be noted that only metal containers can be used for the Bechamp iron reduction in weakly acid solution, for alkali or polysuffide fusions, or for operations under high pressure. Pressure reactions involving very small quantities of materials can be carried out in sealed tubes, however. Except for these applications, metal is used only for apparatus of more than six — or eight-liter capacity. Glass containers are generally satisfactory for all other uses, especially since Pyrex and similar glasses are mechanically strong materials resist* ant to temperature changes and chemical action. Also, they are suitable for the preparation of three — or five-necked flasks or more complicated apparatus.
The use of transparent glass apparatus in the laboratory has the great advantage that the reaction can be observed throughout its course. This is a particularly important factor when a reaction is being run for the first time. On the other hand, it is worthwhile for the technical chemist to become accustomed in the laboratory to working with non-transparent apparatus modelled after plant equipment and to controlling the course of the reaction by removing test samples, just as it must be done on a large scale.
In school laboratories, it is customary to use bent glass rods as stirrers, similar to those shown in Figure 19 (page 101) and Figure 20 (page 105). These stirrers, which are easily prepared by the individual, are quite suitable for mobile liquids, but are not satisfactory for pasty mixtures. With the latter, stirrers presenting more flat surface must be used. One form frequently used in open containers in industrial laboratories consists of a rectangular plate of thick, ribbed glass held in position in a forked holder by two setscrews. These stirrers are easy to keep clean and give a vigorous stirring which can be regulated by changing the angle at which the blade is set. They are, however, quite fragile.
Stirring paddles of porcelain, recently introduced, are less fragile and very satisfactory. They are simply attached to a bent glass rod and are available in various shapes and sizes (Fig. la-c).
A propeller-type stirrer is of value for stirring up mixtures containing heavy solids such as iron powder or zinc dust. Such stirrers, an example of the container and should be large enough to nearly cover the bottom, of which is shown in Figure 17 (page 94), should reach to the bottom. Anchor — or paddle-type stirrers are also useful under these conditions.
Closed reaction kettles, as a rule, have their stirrers built in, usually of the anchor-type which reach nearly to the bottom and sides of the kettle.
|
(a) (b) (c) Fig. 1. Porcelain stirrers for attaching to a bent glass rod. |
It is difficult to get efficient stirring in a glass flask (round-bottomed or three-necked flasks, etc.) because the relatively narrow neck prevents the introduction of a sufficiently broad stirrer. Special types of stirrers have been devised for this purpose in order to achieve vigorous stirring with a stirrer of small diameter. Propeller stirrers (Fig. 6a) centrifugal stirrer, and others, belong to this group. All of them give vigorous stirring at high speed as long as the mixture being stirred is a homogeneous, mobile liquid, but they fail in viscous or pasty mixtures. With the latter, the best results are usually obtained with a simple glass paddle (Fig. 6b) which can be made by pressing out a rolled-up glass spiral.
It should be pointed out that effective stirring is not possible if the container is too full. In open containers of large diameter, such as dishes or flat vessels, the stirrer may set up waves which are thrown over the edge. To prevent this spilling, a “wave-breaker” must be installed, generally consisting of a vertical, thick glass rod near the edge of the container. For the construction and use of autoclaves, see Section M, page 350 ff.
 31 августа, 2015
31 августа, 2015  Pokraskin
Pokraskin 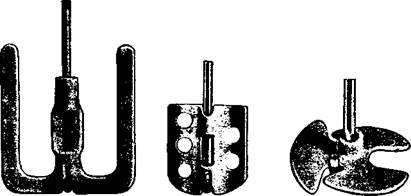
 Опубликовано в рубрике
Опубликовано в рубрике 