|
SO3H SO3H
SOaH SO3H Freund acid Monoazo dye (valueless) |
|
SO3H
|
One-tenth mole of Freund acid (mol. wt. 325; acid sodium salt, C10H8O(jS2NNa) is dissolved in 300 cc. warm water containing 5.6 grams of soda ash. (Freund acid is usually only 75 to 80 per cent pure since it must be precipitated with large amounts of salt because of its high solubility.) The solution is allowed to cool to about 30°C., and enough ice is added to lower the temperature to 0°. 30 cc. concentrated hydrochloric acid is then added. Usually, part of the sulfonic acid is precipitated. With good stirring, a solution of 7 grams of technical sodium nitrite is added over a period of 5 to 10 minutes, keeping the temperature below 6°. The diazotization is complete when the tests for acid (Congo red) and nitrous acid (starch-iodide) persist for 5 minutes.
Freund acid, like many other amines with negative groups (NOo, SO3H, Cl), gives a positive test with starch-iodide after a short time, even though diazotization is incomplete. When diazotization is complete, however, the test should appear within one-tenth of a second. The test with the sulfone reagent (footnote, page 243) is easier for the beginner.
A solution of 14.3 grams of pure a-naphthylamine in 12 cc. concentrated hydrochloric acid and 200 cc. boiling water is allowed to cool to 50°C. with continuous stirring. Usually, a small amount of the hydrochloride separates. This amine solution is poured into the diazonium solution in a thin stream, keeping the temperature of the mixture below 5° by the addition of finely crushed ice. The mixture is stirred for 3 horns, and then a solution of 10 grams of soda ash is added over a period of 2 hours. The reaction mixture is then allowed to stand overnight. On the following day, 30 grams of concentrated hydrochloric acid is added, the temperature is lowered to 0° by the addition of about 300 grams of
ice, and the monoazo dye is diazotized by adding a solution of 6.8 grams of sodium nitrite in 50 cc. water. The diazotization requires about 15 minutes at 0-5°.
The test for complete diazotization is made,* and then the solution is treated with 14.3 grams of a-naphthylamine exactly as described above, again adding 10 grams of soda ash after 3 hours. After an additional 5 hours, a solution of 35 grams of soda ash in 10O cc. cold water is added over a period of 30 minutes, and after 1 hour more the mixture is heated slowly to 80°C. Salt is then added (18 per cent based on the volume). The naphthylamine black precipitates in an easily filterable form and is filtered, pressed, and dried. The dry dye weighs about 70 grams. A 4 per cent dyeing on wool from weak sulfuric acid solution is deep black with some brownish tinge.
Technical Observations. The preparation of naphthylamine black D can be done in various ways. Many industrial chemists prefer to salt out the diazo compound of the monoazo dye and filter it off to purify it. This has the advantage of removing unchanged a-naphthylamine and preventing the formation, in the final product, of the insoluble dye (which is not fast to rubbing):
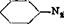 NH,
NH,
“Sudan”-like product
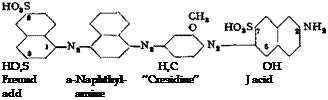 |
The tint of the dye prepared by this method is decidedly purer and stronger. The separation of the diazo compound is especially important when cotton dyes are to be prepared from it, since impurities are especially detrimental in subsequent step. For example, an extremely light fast cotton dye of the Sirius blue type is prepared by coupling the diazo compound with m-amino-p-cresol ether and subsequently coupling this product with J acid:
Direct fast violet R
Direct fast violet RR is formed if m-toluidine is used in place of cresidine (amino — cresol ether).
The diazo compound is dark colored, and usually it is not possible to test the solution directly with stafch-iodide paper. Instead, a drop of the solution or suspension is placed on a small pile of salt resting on a filter paper. The colored materials are precipitated by the salt, and the test papers for mineral acid and nitrous acid are pressed against the back side of the filter paper.
Naphthylamine black is one of the most important azo dyes since it has high color[56] strength and is relatively fast to light as are most of the dyes of the Biebrich scarlet type. It is not entirely fast to boiling and hence its solutions, especially if alkaline, should not be boiled unnecessarily. There is a large number of similar dyes on which data are given in textbooks and in the excellent tables of Schultz. Regarding the manuafcture of these dyes, it should be noted that hydrochloric acid which contains much sulfuric acid should not be used in dissolving a-naphthylamine, since a-naphthylamine sulfate is very insoluble. In place of soda (carbonate), sodium formate can be used for the neutralization in the coupling reaction. The formate is cheaper than sodium acetate.
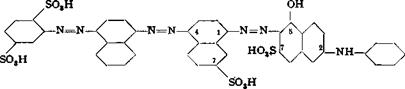
Benzo Light Blue 2 GL or Sirius Light Blue G *
|
(a) Aniline-2,5-dimlfonic Acid — a*Naphthylamine |
The amount of aniline-2,5-disulfonic acid (monosodium salt, see page 106) corresponding to 50 cc. 1 N nitritef is dissolved in 150 cc. hot water, and the solution (filtered if necessary) is treated with stirring with 8 cc. 30 per cent hydrochloric acid. The solution is cooled to 0°C. and 17.5 cc. 20 per cent sodium nitrite solution is added rapidly. The diazotization takes about 15 minutes.
The coupling reaction is carried out most conveniently in the laboratory using an aqueous alcohol solution since a-naphthylamine hydrochloride is rather difficultly soluble.! The diazo solution, therefore, is diluted with an equal volume of 96 per cent alcohol, and a solution of 7.15 grams of freshly distilled a-naphthylamine in 100 cc. alcohol is added very slowly to the well stirred diazo solution. If the addition is made too rapidly, a considerable part of the reactants is occluded by the precipitated dye and made inaccessible for the reaction. The very thick paste is allowed to stand overnight and then the dye is filtered off. It forms a brownish red, bronzy paste, which is not dried for subsequent steps. It must, however, be freed from any residual a-naphthyl — amine. To this end, the dye is dissolved in 300 cc. warm water containing the calculated amount of soda, and the resulting solution is cooled to 0° and held there for 1 hour. The a-naphthylamine separates and is filtered off. The filtrate is used directly in the next diazotization.
 30 ноября, 2015
30 ноября, 2015  Pokraskin
Pokraskin 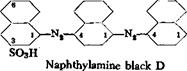
 Опубликовано в рубрике
Опубликовано в рубрике 