A paste is made of the reprecipitated disazo dye with 75 cc. 2 N soda solution and 150 cc. water, the equivalent of 3.5 grams of pure sodium nitrite is added, and the mixture is heated to 80°C. to form a clear solution. The solution is cooled to about 60° and added, over a period of 30 minutes with good stirring, to a mixture of 30 cc. concentrated hydrochloric acid and 200 cc. water. A total of 300 grams of ice is added from time to time to keep the temperature below 8°. The completely clear solution of the diazo compound which is formed is added, in the course of 1 hour, to a solution containing the equivalent of 15 grams of pure phenyl-J acid in 300 cc. water containing 25 grams of soda ash, maintaining the temperature at 0° by the addition of ice. (The large amount of soda is used so that bicarbonate is formed in the coupling reaction and no foaming occurs due to generation of carbon dioxide.) Stirring is continued for 1 hour, and the mixture is then allowed to stand overnight. On the next day, the mixture is heated to boiling, treated with 15 to 20 per cent of salt (based on volume), arid filtered at 80°. The lustrous bronzy paste is washed with 5 per cent salt solution and dried at 100°. The mother liquor is always very deeply colored and contains some phenyl-J acid; this excess is necessary in order to obtain a good yield. The yield of the trisazo dye is about 40 grams. It dyes a pure blue, and, if the dye has been prepared properly, the exhausted dye bath gives the same shade but lighter in tint.
Technical Observations. Dyes of this class are manufactured in the regular equipment for azo dyes as shown schematically in Figure 56, page 376. Because of the instability of the diazo compounds, it is necessary to use large enough filter presses so that the whole batch can be filtered at one time. Then it is possible to work up the diazo compound immediately after the filter press is emptied. It is also advisable to prepare these dyes in the colder parts of the year and to entrust the operations only to reliable workmen. If at all possible, each operation should be piloted by a laboratory run using a small portion (e. g., one-thousandth) of the intermediate from the preceding step. Samples of the individual intermediates should be available in a pure state so that comparisons can be made to show whether the preparation is proceeding normally.
Disaso and Роїуаяо Dyes from Diamines
Bismarck Brown C and R
|
NH.
|
These dyes consist of various high molecular compounds of which the ones pictured above predominate. Procedures given in the literature for the preparation of these dyes are of little value because all of them call for treating an acid diamine solution with sodium nitrite. Much better results are obtained by carefully acidifying a neutral solution of the diamine and nitrite, or by adding the neutral solution, in the course of 12 minutes, to the required amount of hydrochloric acid. Furthermore, it turns out that somewhat more nitrite should be used than is called for by the equation:
3 Diamine + 2 NaN02 + 4 HC1 -» 1 Dye + (2 HC1) + 2 NaCl + 2 H20
The excess used is about 24 per cent in the case of m-phenylenediamine, and about 20 per cent in the case of toluylenediamine. The diamine is then completely used in the dye formation as can be shown by salting out a test portion.
 1 декабря, 2015
1 декабря, 2015  Pokraskin
Pokraskin 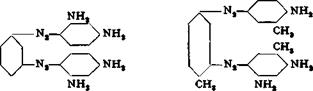
 Опубликовано в рубрике
Опубликовано в рубрике 