10 grams of the dye prepared above is boiled under reflux with 250 cc. water and 25 cc. 2 N sodium hydroxide until solution is complete. The solution is cooled and filtered through a fluted filter. The filtrate is added slowly to a vigorously stirred solution, held at 25-30°C., containing 8 grams of crystalline calcium acetate and 30 cc. 2 N acetic acid in 500 cc. water. Each drop of the solution added should immediately produce a very finely crystalline, shiny red precipitate. When the addition has been completed, the mixture is warmed 80-90° and filtered hot with suction. The precipitate is washed thoroughly with hot water and dried in a steam heated oven. The yield is quantitative.
Remarks. Helio Bordeaux BL, which is rather difficultly soluble even in the form of its sodium salt, gives a completely insoluble calcium salt having a bright blue-red color. This calcium salt possesses, to a liigh degree, the properties required by the color lake industry, and is especially valuable for the preparation of printing inks. Its color purity, however, is adversely affected by the slightest impurity. In order to prevent the formation of such detrimental impurities, it is necessary to carry out the diazotization of the a-naphthylamine in such a way that no trace of aminoazonaphthalene or its diazo compound is formed. This is accomplished by using an unusually large excess of hydrochloric acid. Furthermore, the coupling reaction is not carried out in the usual mannef, using a solution which is alkaline from the beginning. Instead, the acidic mixture of diazo compound and coupler is gradually neutralized; this procedure prevents partial coupling in the 4 position. Since, on mixing the diazo compound and coupler in acid solution, the difficultly soluble a-naphthyldiazonium salt of l-naphthol-5-sulfonic acid separates
in good crystalline form, it is recommended that this salt be filtered off, thus eliminating the excess acid. The conditions under which the sodium salt of the dye is converted to the calcium salt are of real importance in the precipitation of the lake.
The l-naphthol-5-sulfonic acid is prepared90 by alkali fusion, at 160-190°C., of naphthalene — 1,5-disulfonic acid (see page 219); it can also be prepared by the Bucherer reaction from l-naphthylamine-5-sulfonic acid (see page 214).
Single Coupling Reactions with Amines
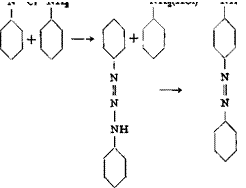 |
p-Aminoazobenzene from Aniline
A solution of 28 grams (0.3 mole) of aniline in 350 cc. 2 N hydrochloric acid is externally cooled to about 20°C. and then to 0° by the addition of crushed ice. The solution is diazotized by the addition of a solution of 22 grams of technical sodium nitrite in 150 cc. ice water. The addition of the nitrite solution can be completed in less than 1 minute. The resulting diazo solution must be clear, any cloudiness indicating lack of sufficient acid. The diazotization is complete after about 10 minutes (Congo red and starch-iodide tests).
To the diazo solution is added a solution made from 31 grams of aniline, 200 cc. water, 100 grams of ice, 40 cc. 2 N acetic acid, and 120 cc. 2 N hydrochloric acid, and the mixture is allowed to stand for 10 minutes. A solution of 50 cc. 25 per cent ammonia in 400 cc. water is then added, with continuous stirring, at such a rate that the reaction mixture stays distinctly acid to litmus at all times. The ammonia should be added below the surface of the reaction mixture. The addition requires 2 to 3 hours, after which the reaction mixture gives no test for diazotized aniline with H acid solution. The yellow diazoamino com-
90Ewer and Pick, Ger. Pat. 41,934 [Frdl, 1, 398 (1877-1887)].
pound precipitates in an easily filterable form. It is filtered off and washed with distilled water. The filter cake weighs 120 to 170 grams.
Rearrangement of the Diazoamino Compound. The precipitate is stirred with 140 grams of aniline. The solid dissolves rapidly, and the water, which was retained in the diazoaminobenzene, separates on the surface of the oil and is removed by a small pipette. 10 grams of aniline hydrochloride is then added, and the mixture is heated slowly, with continuous stirring, to 30°C. on a water bath. After 2 hours, the temperature is carefully raised to 40° (not higher) and held there overnight. On the next day, a small portion of the mixture is heated to 100° in a test tube. If the rearrangement is completed, no nitrogen is evolved and no odor of phenol is detectable. If the reaction is not complete, the mixture is heated for 1 hour at 50°, but this is usually not necessary.
The Oily mixture is poured into a well stirred solution of 200 cc. concentrated HC1 in 300 cc. water. The hydrochloride of amiuoazoben — zene separates as a graphite-like precipitate and is filtered off and washed twice on the funnel with acidified water (4 cc. HC1 in 100 cc. water). The resulting material is quite pure. It can be recrystallized from about 3 liters 1 per cent hydrochloric acid. In practice, the dilute acid from this recrystallization is used for three or four successive batches in order to minimize losses. It is often observed that the amino — azobenzene hydrochloride separates first as red crystals which are rapidly converted to the graphite-like form. The pure salt, dried at 50- 55°, weighs 49 to 50 grams, which is about 70 per cent of the theoretical amount.
The free base can be prepared by digesting the hydrochloride with dilute soda solution or ammonia. Generally, however, the compound is used in the form of its hydrochloride.
Aminoazotoluene is prepared by the same method in even better yields.
Fast yellow is the disulfonic acid of aminoazobenzene. The first sulfonic acid gtoup enters predominantly the position para to the azo group, yielding a yellow wool dye which has only moderate fastness. When a second sulfonic acid group is introduced, it is forced to enter a position ortho to the amino (or azo) group, and the resulting dye has very much higher light fastness.
This sulfonation is effected very easily. Dry aminoazobenzene hydrochloride is added to 3 parts of 25 per cent oleum, and the mixture is stirred at 25°C. until a test sample dissolves in soda solution. The temperature is then raised to 40° and the mixture is held at this point, with good stirring, for about 5 hours or until a test sample dissolves completely in a large volume of water. The mixture is poured onto 6 parts of ice, and the monosodium salt of the disulfonic acid is salted out by the addition of 200 grams of salt per liter of solution. The flesh-colored precipitate is filtered off and washed thoroughly with 15 per cent salt solution, and the filter cake is then stirred with a small amount of water at 50°. Enough soda is added to make the color of the solution a pure yellow. The amount of soda required depends on the thoroughness of washing. The fast yellow need not be salted out, but the solution can be evaporated to dryness at 90°. The product weighs about twice as much as the starting material.
Aminoazobenzene is prepared industrially in large enameled kettles of 300- to 400-liters capacity. The reaction mixture is acidified in the usual wooden vats and the aniline-containing mother liquor is worked up to recover the aniline (about 15 per cent loss) by adding lime and steam distilling. Claims to the contrary notwithstanding, fast yellow is not as fast to light as tartrazine and is much less fast than the pyrazolone dyes which have a sulfo group ortho to the azo group.
Aminoazobenzene is an important starting material for many disazo dyes. When it is diazotized and coupled with phenols and other couplers, secondary disazo dyes are formed. The first of these dyes, that from aminoazobenzenedisulfonic acid ‘and /3- naphthol, was Biebrich scarlet, and the whole group of dyes is generally referred to as the Biebrich scarlet type. Diazotization of aminoazobenzene requires several hours. The freshly prepared hydrochloride is suspended in 5 parts of water, and 150 gram; of hydrochloric acid is added for each mole of the salt. A small test run should be made at greater dilution to determine the amount of nitrite required. The diazotization is done at 10-14°C., and large scale preparations frequently require a whole day. The completed diazotized mixture is either used immediately or is cooled to 0° by the addition of ice.
Aminoazobenzene, like aniline, can be condensed with dinitrochlorobenzene. The product is phenylazodinitrodiphenylamine, an insoluble, beautifully crystalline material which can easily be converted to the monosulfonic acid by the action of 100 per cent sulfuric acid. This nitroazo dye (azo flavine FF) is very similar fn structure to azo yellow G, described on page 276, but is superior to the latter in that it is a more homogeneous product and does not lose nitrous acid on heating. For these reasons, it is preferred by some silk dyers over the ordinary azo yellow even though it is more expensive.
Azo flavine FF:
|
МП МП
SO. H |
Phenylazodini — Azo flavine FF trodiphenyl — amine
(a) Condensation of Aminoazobenzene with Dinitrochlorobenzene■ A mixture of 100 grams (dry weight) of moist aminoazobenzene hydrochloride, 100 grams of dinitrochlorobenzene, and 250 grams of crystalline sodium acetate in 600 grams of 90 per cent alcohol is heated under reflux, with stirring, for 6 hours. The condensation product separates as reddish brown, glistening crystals which are filtered from the hot solution and vyashed with alcohol. The material, after drying at 100°C., weighs about 115 grams.
out without alcohol, but the product contains so much unreacted di — phenylamine that the subsequent nitration of the dye is difficult, is insoluble in water so the coupling reaction must be done in water — alcohol mixture. Under certain conditions, the reaction can be carried (b) Sulfonation. One part of the condensation product and three parts of 100 per cent sulfuric acid are stirred at 30°C. for 1 hour. The temperature is then raised slowly to 45° and held at this point for 1 to 2 hours, until a test portion of the mixture is poured into 6 parts of water, and the dye is salted out, filtered off, and washed with 15 per cent salt solution. The acid is then dissolved in a small amount of hot water containing the necessary amount of soda. From this solution, the sodium salt is sidted out by adding 15 per cent of salt (based on volume). The precipitate is gelatinous at first but rapidly becomes crystalline and easily filterable. The yield of dye from 100 grams of condensation product is about 125 grams.
Azo flavine FF has about the same tint as tropaeoline and the high acid stability of the highly nitrated azo yellow.
 26 ноября, 2015
26 ноября, 2015  Pokraskin
Pokraskin 
 Опубликовано в рубрике
Опубликовано в рубрике 