Monastral Fast Blue BS, Heliogen Blue В
|
|
In a 500-cc. 3-necked flask, surrounded by an oil bath and equipped with thermometer, stirrer, and vertical tube, are placed 37 grams (0.25 mole) of phthalic anhydride, 40 grams of urea, 0.2 gram of ammonium molybdate, and 260 grams of 1,2,4-trichlorobenzene. The mixture is stirred vigorously and heated, during the course of 1 hour, to 195°C.
(internal temperature, oil bath temperature about 220°). This temperature is maintained as closely as possible for 4 hours while stirring is continued, and during this time 10 grams of cuprous chloride is added gradually in small portions. Care must be taken that the vertical tube does not become stopped up by sublimed phthalic anhydride, the accumulated sublimate being melted down from time to time. The mixture is then cooled, and the resulting viscous greenish-blue paste is filtered on a sintered glass funnel. The red-violet, crystalline precipitate is washed successively with hot alcohol, hot 2 N hydrochloric acid, hot 2 N sodium hydroxide, and finally with hot water, the washing with each solvent being continued until the filtrate is colorless. The product is then dried, yielding a finely crystalline, violet-blue powder weighing 27 to 28 grams (75 to 78 per cent of the theoretical amount).
Remarks. The copper phthalocyanine was first obtained by de Diesbach[69] in the course of an attempt to prepare phthalonitrile from
 |
 |
o-dibromobenzene by heating with cuprous cyanide. Independently, and a short time later, it was observed by Scottish Dyes that a blue dye was formed in the preparation of phthalimide in an iron container. Extensive research, especially by Linstead [70] and collaborators, showed that this blue dye was an iron derivative of the same compound obtained earlier. Linstead named the parent substance phthalocyanine and pro
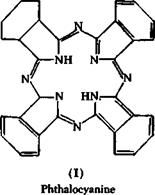
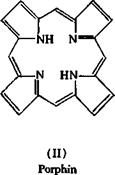
posed structure I for it. This structure is strikingly analogous to that of porphin (II), which is the basic structure of hemogoblin and chlorophyll. Linstead established that phthalocyanines are easily formed when phthalonitrile or other o-dinitriles, or any compound or mixture
which yields o-dinitriles on heating, are heated to higher temperatures in the presence of suitable metals or metal compounds. The phthalocy — anines have a strong tendency to form complex compounds with numerous metals. The copper compound, especially, is characterized by an extraordinarily high stability. It can be sublimed unchanged at about 600° in vacuum. It can be boiled with dilute acids or caustic alkali, and can even be dissolved in cold, concentrated sulfuric acid, without being decomposed. It is completely insoluble in water and in the usual organic solvents and is extraordinarily fast to light. Thus, the compound pos — essses, to the highest degree, all the properties required for a pigment color.

 26 декабря, 2015
26 декабря, 2015  Pokraskin
Pokraskin 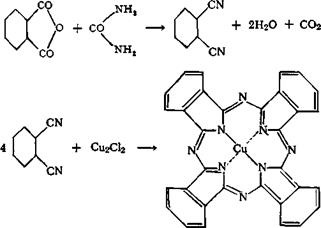
 Опубликовано в рубрике
Опубликовано в рубрике 