The diagram in Figure 28 best explains the procedure. Naphthalene is placed in flask B, which is heated to 110°C., and a stream of moist air is passed through the gas introduction tube (A). The presence of water vapor is important for the oxidation. The necessary air is most
|
Fig. 28. Apparatus for preparation of phthalic anhydride by catalytic oxidation: A, air inlet tube; B, melted naphthalene; C, graphite bath; D, electrical heating element; E, ground; F, spark induction coil; G, glass wool; H, catalyst; J, bulb receier; K, aluminum foil, grounded; L, gas exit tube; M, thermocouple; N, rigid wire; Th, thermometer. One side of the spark induction coil is connected to N, the other is grounded. |
simply supplied by a water pump which is readily available in the laboratory, and the quantity of air passed through the apparatus is measured with regular laboratory equipment which need not be described here. The air stream should be regulated so that 200 liters of air and 5 grams of naphthalene are passed through the apparatus in 1 hour. It is important that the ratio of air to naphthalene be controlled so that an explosive mixture is not formed, since this would cause a loss of phthalic anhydride. The mixed gases (air and naphthalene vapor) are passed into the catalyst tube, which should be of about 5-cm. internal diameter and should contain a charge of catalyst about 10 cm. in length. The pumice particles are held in place by glass wool. Heating of the catalyst is done electrically as is the usual practice in technical laboratories.
The gases coming from the catalyst are passed first into a round receiver (J), and then go to the precipitator. The latter consists of a glass tube of about 8-cm. internal diameter, the inside wall of which is lined with a smooth aluminum foil which can be removed easily and which is well grounded through a metal wire to a water pipe. A wire or silvered glass rod projects into the tube, and to this a potential of 10,000 volts is applied. This potential, which can be alternating for the present purpose, is generated in the usual manner, most simply by an induction coil or a transformer with interrupter. It is important that the air spark length of the induction coil be at least 25 mm. The internal width of the tube carrying the precipitator must therefore be sufficiently great so that sparks do not jump from the wire to the metal foil. Such sparking may cause explosions which, although not involving danger, may easily bum part of the reaction product.
When the apparatus, to which careful attention must be given, is assembled correctly, the oxidation can be started. The catalyst tube is heated to 450°C. (thermocouple), and the stream of mixed air and naphthalene vapor is started. The oxidation takes place as the vapors pass through the catalyst. Part of the phthalic anhydride collects in the bulb receiver, but most of it is collected in the Cottrel precipitator where it frequently forms beautiful needles, up to 5 cm. in length, in a small space at the extreme first part of the precipitator. The operation can be continued as long as desired, since the catalyst retains its activity for weeks provided that pure naphthalene is used. The yield of phthalic anhydride is about 90 to 95 per cent of the theoretical amount, the product being chemically pure if the oxidation temperature is maintained at the correct point and the throughput is not too high. If too much naphthalene is put through per unit of time, the product becomes yellowish due to admixed 1,4-naphthoquinone. The product is removed from time to time by drawing out the aluminum foil. 20 grams of pure phthalic anhydride can easily be prepared in 5 hours in the laboratory.
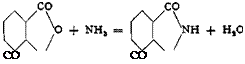 |
Phthalimide from Phthalic Anhydride
In a three-necked flask fitted with thermometer, gas introduction tube, and wide outlet tube bent downward, 148 grams (1.0 mole) of pure phthalic anhydride is melted and heated to about 170°. At this
temperature a rapid stream of ammonia is passed into the molten anhydride. The ammonia is completely absorbed and steam escapes, carrying with it some phthalic anhydride. Sublimed material which condenses in the neck of the flask is melted down from time to time. During the introduction of the ammonia, the temperature is raised slowly until it reaches 240°. At this point, the phthalimide does not solidify since its melting point is 230°. When ammonia begins to escape, the reaction is nearly complete. The stream of ammonia is continued for 10 minutes more, and the melt is immediately poured into a porcelain dish where it is allowed to solidify. About 130 to 135 grams of phthalimide is obtained, melting at 223° (uncorrected) and containing as an impurity only traces of unchanged phthalic anhydride.
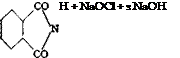 |
|
Anthranilic Acid from Phthalimide
A solution is made by stirring 73.5 grams (0.5 mole) of finely powdered phthalimide with 250 cc. water and 125 grams of ice and then adding 55 cc. sodium hydroxide solution (40° Вё). As soon as solution is complete, 250 grams of ice is added and then, in one portion, a chlorate-free sodium hypochlorite solution, which corresponds to 37 grams of active chlorine and contains two moles of NaOH for each mole of NaOCl.* An additional 20 cc. 40° Вё sodium hydroxide solution is added just’ before use. A drop of the reaction mixture added to a few drops of aniline water should give only a very weak violet coloration. After the solution is thoroughly mixed, it is allowed to stand in an ice bath for 3 to 4 hours, and then a few drops of bisulfite solution are added to destroy the small excess of hypochlorite. The solution is then heated to 80° and neutralized at this temperature with concentrated hydrochloric acid (foaming) just to the point where litmus paper is not turned blue (about 130 cc. acid). The hot solution is filtered to remove any inorganic impurities, and the filtrate is acidified by adding 40 cc. concentrated hydrochloric acid and 12 cc. glacial acetic acid. (An
* The hypochlorite solution is easily prepared by adding 71 grams of chlorine to a mixture of 400 cc. sodium hydroxide solution (40° Be) and 400 grams of ice, cooled in an ice bath. The concentration of active chlorine is determined iodo- metrically. Commercial hypochlorite solutions, which have been held for a long time, contain appreciable amounts of chlorate and are not suitable for the present purpose.
excess of hydrochloric acid must be avoided, since it would dissolve the anthranilic acid.) Anthranilic acid begins to separate from the hot solution as a crystalline, light brown precipitate becoming more copious on cooling. After standing overnight, the product is filtered off, washed with cold water, and dried in a steam heated drying oven. The yield of anthranilic acid melting at 144-145° is 57 to 58 grams, or about 84 per cent of the theoretical amount.
The anthranilic acid remaining in solution in the filtrate can be recovered by adding copper acetate to form the insoluble copper salt. This salt is filtered off, suspended in water, and decomposed by passing in hydrogen sulfide. The copper sulfide is filtered off and the filtrate is concentrated to a small volume. When the resulting solution is cooled, a few grams of anthranilic acid crystallize out.
 15 октября, 2015
15 октября, 2015  Pokraskin
Pokraskin 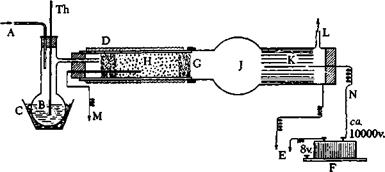
 Опубликовано в рубрике
Опубликовано в рубрике 