Gallamine Blue from Gallamide
Nitrosodialkylanilines react with gallic acid or gallamide under the influence of heat to form well defined compounds designated as oxa — zines. The gallic acid is obtained exclusively from natural tannins.
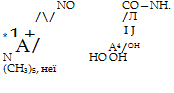 |
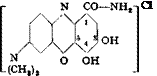 |
p-Nitrosodi — Gallamide Gallamine blue
methylaniline
(One molecule of nitrosodimethylaniline serves as
an oxidizing agent.)
(a) Nitroiodimethylaniline
A mixture of 100 grams of dimethylaniline and 200 grams of 30 per cent hydrochloric acid is cooled, and 300 grams of ice is added. The
* At this point, the solution should not change color from blue to green; if it does, too much acid has been added. Excess acid prevents complete precipitation of the dye and should be neutralized by the addition of sodium acetate.
beaker containing the mixture is placed in an ice bath, and a solution of 60 grams of 100 per cent sodium nitrite in the least possible amount of water is added dropwise over a period of 5 hours. The presence of free nitrous acid in the reaction mixture cannot be tested for by starch — iodide paper, since nitrosodimethylaniline itself gives a positive test. Excess nitrous acid, therefore, must be detected solely by smell. The mixture should have an acid reaction to Congo red, of course. After 6 hours, the mixture is filtered, and the precipitate, rinsed into the funnel by some of the mother liquor, is sucked as dry as possible, pressed out in a screw press, and then pulverized. The resulting nitrosodimethylaniline hydrochloride should not be dried, but used while still moist. In large scale operations, the product is dried sufficiently merely by centrifuging.
p-Nitrosodiethylaniline is prepared in a similar way, except that no water is added to the nitrosation mixture because of the very high solubilty of the hydrochloride. Concentrated hydrochloric acid and saturated sodium nitrite solution are used. Industrial preparations are carried out in enameled equipment such as is used in tropaeoline coupling.
 9 декабря, 2015
9 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 