In the naphthalene series, two isomers, a and /3, are possible wuen only one substituent is present. Nitration and halogenation of naphthalene give practically only the a compound. On the other hand, sulfonation with concentrated sulfuric acid always gives a mixture of the two isomers (pure a-naphthalenesulfonic acid can be obtained by sulfonation with chlorosulfonic acid in an organic solvent), the relative amounts of the two depending on the sulfonation temperature. In the cold, the chief product is the a-sulfonic acid, while at temperatures above 120-130°C., the ^ sulfonic acid predominates. It might be emphasized here that the naphthalenesulfonic acids, once formed, frequently undergo rearrangement when heated with concentrated sul-
8 Akt.-Ges. Anilin-Fab. Berlin, Ger. Pat. 157,859 (1904), 164,130 (1905), and 163,516 (1905) [Frdl., 8, 104-110 (1905-1907)1
furic acid or oleum, forming finally an equilibrium mixture of the various isomers, the composition of the mixture being dependent on the temperature.
The introduction of a second substituent, and of subsequent ones, is governed by the rules holding in the benzene series, but in greatly modified form. The modified rules may be summarized as follows.
Strongly orienting groups of Class 1 (hydroxyl, alkoxyl, and amino or acylamino groups in the absence of concentrated sulfuric acid), when present in the 1 position of the naphthalene nucleus, direct the entering substituent to the otfho or para position (2 and 4 positions) just as in the benzene series. Less active groups of Class 1 (halogen, and amino in the presence of concentrated sulfuric acid) often give rise to the 5 isomer, instead of the 2 isomer, along with the 4 substituted compound.
Groups of Class 1, when present in the 2 position, direct partly to the 1 position (in direct relation to the strength of their orienting influence), and partly to positions in the other ring, especially the 8 position, then the 6 position. Sulfonation of /З-naphthylamine gives a mixture of all four heteronuclear monosulfonic acids. The 3 position is seldom entered, and then only if the other ring bears at least one substituent. The reaction of carbon dioxide with sodium /З-naphtholate is an exception to this rule. In this case 2-hydroxy-l-naphthoic acid is formed at lower temperatures, but at higher temperatures the product is 2-hydroxy-3-naphthoic acid.
Groups of Class 2 favor the entrance of a second substituent into the other ring almost exclusively; the meta position of the same ring is usually entered only if there are other substituents already present in the other ring.
Another rule holding generally for the introduction of the second and subsequent substituents is that halogen and nitro groups usually enter the a position, while sulfo groups enter predominantly the a position at lower temperatures and the /3 position at higher temperatures.
Of greater importance is the rule stated by Armstrong and Wynne, governing the course of sulfonation reactions. This rule says that in direct sulfonation reactions, sulfo groups never enter positions ortho, para, or peri to each other. However, rare and technically unimportant exceptions to this rule are possible under the influence of other strongly directing substituents, but never where substitution by sulfo groups alone is concerned. Thus, the almost unlimited number of possible isomers is greatly reduced, and sulfonation proceeds according to the following scheme:
|
|
Further nitration of a-nitronaphthalene leads almost exclusively to
1,5- and 1-8-dinitronaphthalenes.
In the nitration of naphthalenesulfonic acids, the a position of the non-sulfonated ring is attacked preferentially. If a sulfo group is present in each ring, the entering nitro group seeks out whichever a position is not ortho or para to a sulfo group. If no such position is available, mixtures are formed; in part, the nitro group enters a position para to a sulfo group, and in part it takes a /3 position meta to a sulfo group. Naphthalene-1,3,5,7-tetrasulfonic acid cannot be nitrated.
Concerning the sulfonation of naphthols and naphthylamines, see the discussion under the individual compounds.
3. Orientation in the Anthraquinone Seriet
The relationships in the anthraquinone field are somewhat more complicated than those in the benzene and naphthalene series.
In contrast to benzene and naphthalene compounds, anthraquinone can scarcely be sulfonated with concentrated sulfuric acid because the temperature required is so high that the anthraquinone is largely destroyed. Therefore, fuming sulfuric acid must be used, permitting a lower reaction temperature. Under these conditions, it turns out that it is not possible to introduce a single sulfo group, but a second sulfo group is also introduced. In order to obtain a reasonably pure mono — sulfonic acid, it is necessary to conduct the sulfonation so that only about 50 per cent of the anthraquinone is attacked. Even under such mild conditions, appreciable quantities of disulfonic acids are formed.
When anthraquinone and ordinary fuming sulfuric acid are used, the sulfo groups enter the /3 positions almost exclusively, as shown in the following structural formulas:[4]
|
CO , УУ80-" |
![]()
|
![]()
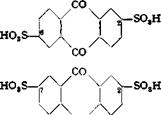
About 50 per cent each of the 2,6- and 2,7-disulfonic acids is formed.
If sulfonation is carried out in the presence of mercury salts, the first sulfo group enters the a position preferentially. If the sulfonation is then carried to the disulfonic acid, the relationships become very complicated, and at least four isomers are formed: the 1,5, 1,8, 1,6, and 1,7 isomers. These isomers can be separated very satisfactorily by keeping the reaction mixture cold (under some conditions with the
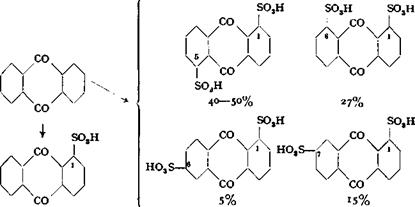 |
addition of a little water), whereupon the 1,5-disulfonic acid separates out almost quantitatively. On further dilution, the 1,8 compound precipitates, leaving the 1,6 and 1,7 isomers in the sulfuric acid solution.[5]
In the anthraquinone series, the a-sulfonic acids are characterized by the fact that the sulfo group is easily split out and replaced by nascent chlorine (also bromine). a-Chloroanthraquinone may be obtained in this way in excellent purity and yield. The /З-sulfonic acids also are capable of undergoing this reaction, but the reaction goes very slowly and not quantitatively because, besides replacement of the sulfo group by halogen, some replacement by hydrogen takes place also.
Nitration of anthraquinone proceeds in a manner analogous to sul-
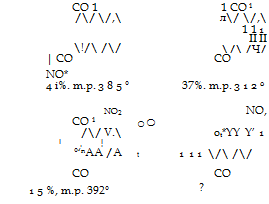 ,CO
,CO
/ /’ CO
fonation, except that mercury salts have no effect on the orientation. A technically satisfactory preparation of mononitroanthraquinone has not yet been found.[6] The formulas just preceding give the pertinent facts about the dinitroanthraquinones.[7] Here again, the 1,5 derivative is the most difficultly soluble and can be easily obtained pure.
Nitration of anthraquinonesulfonic acids follows the same general pattern as in the naphthalene series. A mixture of various isomers is always formed, and of these the 1,5 derivative is easily separated, the others with more difficulty.
Animation of anthraquinone can be accomplished in various ways. Either the sulfo group is replaced by the —NH2 or the corresponding nitro compound is reduced. The reduction of nitro compounds in this series takes place very smoothly by heating with sodium sulfide solution. The formation of a green hydroxylamine derivative, soluble in sodium sulfide solution, is always observed as an intermediate step, after which the pure amine separates as a red crystalline precipitate which needs only to be washed. In the replacement of the sulfo group by —NH2, effected with ammonia at high temperature (hence, under pressure, see pages 229 and 231), it is necessary to remove the ammonium sulfite formed in the reaction in order to prevent extensive reductive destruction of the anthraquinone derivative. For this purpose, reagents are used which either oxidize the sulfite, such as pyrolusite, arsenic acid, m-nitrobenzenesulfonic acid, and many others, or precipitate it as the alkaline earth or magnesium salt so that it is harmless (see page 230). These aminations do not proceed quantitatively, and today, for example, /J-aminoanthraquinone is usually prepared from 2-chloroanthraquinone, which in turn is easily prepared from phthalic anhydride and chlorobenzene.[8]
The introduction of an hydroxyl group into the anthraquinone molecule is accomplished by heating the corresponding sulfonic acid with an alkaline earth hydroxide, whereby again the harmful sulfite is precipitated. If caustic alkali is used, undesired side reactions occur which lead, under certain conditions, especially in the presence of oxidizing agents, to more highly oxidized anthraquinone derivatives (see alizarin, page 314). Quinizarin, i. e., 1,4-dihydroxyanthraquinone, is prepared most satisfactorily today by condensing phthalic anhydride with p-chlorophenol in th’e presence of concentrated sulfuric acid,
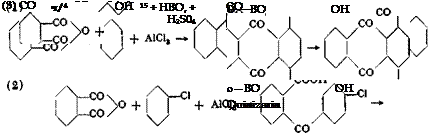
usually also in the presence of boric acid which transforms the quinizarin into the boric acid ester, thus preventing its further reaction.14
Further transformations of the anthraquinone derivatives mentioned above lead to very complicated relationships under some conditions, and these can be discussed only briefly here.
1- Aminoanthraquinone can be sulfonated relatively easily in the 2 position by treating it with fuming sulfuric acid in the presence of bisulfate. The l-aminoanthraquinone-2-sulfonic acid thus formed can also be prepared smoothly by baking the acid sulfate of 1-amino — anthraquinone at 200°C.1 b’ It is a very important starting material for the preparation of valuable acid anthraquinone dyes. lea
Instead of the amino group, other amine residues can be introduced. Of special importance are the dianthraquinonylamines, which can be converted by condensation into brown carbazole-anthraquinone vat dyes. This condensation is usually brought about by aluminum
>« See p. 237 and Bayer & Co., Ger. Pat. 255,031 (1912) [Frtfl., 11, 588 (1912- 1914); C. A., 7, 1587 (1913)1 Also Murch, U. S. Pat. 1,746,736 (1930) [C. A., 24, 1651 (1930)1. General literature: Houben, Das Anthracen und die Anthrar chinone, Thieme, Leipzig, 1929.
15 Or p-chlorophenol (Ger. Pat. 255,031, see footnote 14).
18Cf. Huber, Dissertation, Zurich, 1931, p.49.
18a Fierz-David, Kiinstliche organische Farbstoffe, Erganzungsband. Springer, Berlin, 1935, p. 77.
chloride, less often by aluminum sodium chloride or aluminum chloride and pyridine. These reactions are described in an interesting book by Kranzlein,[9] along with a large number of other syntheses which are very important technically, such as the preparation of dibenzopyrene — quinone (indanthrene golden yellow GK) from benzanthrone and benzoyl chloride in the presence of aluminum chloride and oxygen.
Halogenation of anthraquinone takes place only with difficulty, but the hydroxyl and amino derivatives are easily halogenated, particularly with bromine. Thus, 1-aminoanthraquinone is brominated smoothly in glacial acetic acid to yield l-amino-2,4-dibromoanthraqui — none, an important starting material for the preparation of beautiful acid wool dyes (page 232).
For the sake of completeness, the so-called Bohn-Schmidt reaction should also be mentioned. This reaction is an oxidation of anthraquinone with fuming sulfuric acid or, under some circumstances, the oxidation of the nitro derivative in the presence of sulfur and boric acid. At the present time, these reactions are of little importance technically.[10]
Because of the high reactivity of anthraquinone toward various reagents, reactions are possible in this series which are unknown in the benzene and naphthalene series. Two examples might be mentioned: the indanthrene fusion, whereby two anthraquinone molecules are joined through two —NH— groups, and the dibenzanthrone fusion, in which two dibenzanthrone molecules are joined to form important vat dyes. This heightened reactivity often appears to an even greater degree in the derivatives of anthraquinone, as, for example, in the preparation of quinizarin green base (page 317).
 15 сентября, 2015
15 сентября, 2015  Pokraskin
Pokraskin 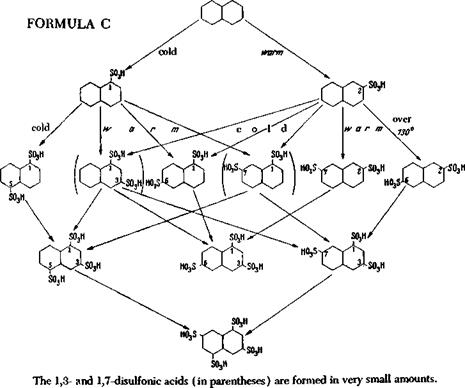
 Опубликовано в рубрике
Опубликовано в рубрике 