In a three-necked flask, provided with calcium chloride tube, dropping funnel, and thermometer, the 130 grams of chlorination product from above is stirred with 2400 grams of 20 per cent oleum for 1 hour at 100°C. The mixture is then cooled to 70°, 1300 grams of concentrated sulfuric acid is added, and the solution is heated to 120° for 30 minutes. The resulting reddish brown solution is again cooled to 70°, and 260 grams of nitric acid (sp. g. 1.52) is added over a period of 1 hour. The temperature rises from 70 to 130° and nitric oxides are given off. At the end of the reaction, the solution is heated for a short time at 160°, during which it becomes much lighter in color. The mixture is cooled, poured into 10 liters of water (copious evolution of nitric oxide), and allowed to stand overnight. The yellowish product is filtered off, washed with water, and taken up in 1 liter of 2 N soda solution which dissolves almost all of the material. The brownish yellow solution is treated with animal charcoal, heated briefly, and filtered. Acidification of the filtrate with concentrated hydrochloric acid precipitates pure, white naphtha- lenetetracarboxylic acid, which is converted to its anhydride by drying for 3 hours at 110°. The yield is 60 to 70 grams, or 45 to 52 per cent of the theoretical amount based on pyrene.
Technical Observations. Naphthalenetetracarboxylic acid is obtained most easily — analogously to phthalic acid — by oxidation of the corresponding higher ring system, pyrene. Instead of first chlorinating the pyrene, it can be oxidized by dichromate and sulfuric acid to form pyrenequinone (mixture of the 3,8 and 3,10 quinones) and this can then be oxidized further to naphthalenetetracarboxylic acid by chloride of lime in the presence of lime, according to the reaction:
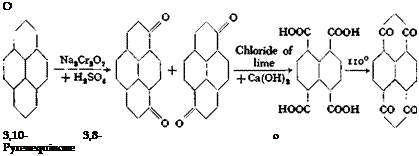 |
This procedure is more suitable for large scale operation, giving a yield about equal to that of the first process.
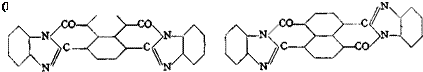 |
Naphthalenetetracarboxylic acid and its anhydride react, similar^ to phthalic anhydride, with o-diamines to form intensely colored bis-imidazoles. These compounds, in contrast to the corresponding derivatives of phthalic anhydride, have the properties of vat dyes. When o-phenylenediamine is used, a mixture of the two isomers, I (cts) and II (trans), is formed. This mixture is known in the trade as indanthrene scarlet 2G. The trans isomer (II), isolated from the mixture, is known as indanthrene brilliant orange GR.
Indanthrene Scarlet 2G
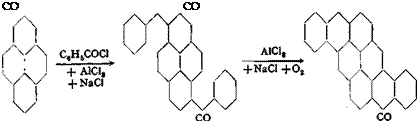 |
Pyrene is one of the high molecular hydrocarbons which have been isolated, in recent years, on a commercial scale from the highest boiling fractions of coal tar (and also from the products of coal hydrogenation).[45] This new starting material has rapidly found a widespread use,[46] particularly in the synthesis of vat dyes. Thus, 3,8-dibenzoylpyrene, on heating with aluminum chloride,[47] yields pyranthrone, which was formerly prepared from p-methylanthraquinone:
Benzpyrenequinone, which also contains the pyrene nucleus, and which is a valuable vat dye, is not prepared from pyrene, but from benzanthrone.
 4 ноября, 2015
4 ноября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 