The formation of methylene blue is interesting both scientifically and technically, and should be reviewed briefly before the actual procedure is described.
(a) Nitrosodimethylaniline is prepared from dimethylaniline by treament with nitrite in acid solution. The nitroso compound is then reduced to form p-aminodimethylaniline.
NO NH2
(b) The p-aminodimethylaniline is oxidized in acid solution with another molecule of dimethylaniline, and simultaneously a thiosulfonic acid group is introduced. This step is accomplished by oxidizing in the presence of thiosulfuric acid in statu nascendi.
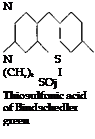 NH.
NH.
N S
(CH3), I
SO. H
Thiosulfonic acid of p-amino-
dimethylaniline
(c) The thiosulfonic acid is then oxidized further and undergoes ring closure to form methylene blue.
![]()
![]()
![]()
![]()
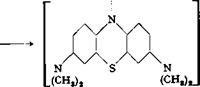 N.
N.
N S
(CHS)3 I
A cooled solution of 24.2 grams (0.2 mole) of pure dimethylaniline in 75 grams of 30 per cent hydrochloric acid is mixed with 150 grams of ice,
and a 20 per cent solution containing 14.7 grams of sodium nitrite is added over a period of 1 hour. Nitrosation is complete in 4 hours. To the solution are added 110 grams of 30 per cent hydrochloric acid and 200 grams of ice, and then, over a 15-minute period with thorough mechanical stirring, 35 grams of high quality zinc dust. The temperature may rise to 25°C. without causing damage. The solution, which becomes colorless and neutral, is filtered and the residue of zinc dust is washed with a small amount of water.
This oxidation must be carried out in the presence of a zinc chloride solution which has no reducing action. A suitable solution can be prepared by dissolving sheet zinc in concentrated hydrochloric acid. (In the industry, the technical zinc chloride liquor is treated with enough sodium bichromate to destroy the reducing action. Frequently, this requires 100 to 250 grams of bichromate for 100 kilograms of the liquor.) The thiosulfuric acid is supplied by aluminum thiosulfate which is so highly dissociated that it reacts as free thiosulfuric acid.
Before the preparation of methylene blue is started, solutions of the necessary reagents are prepared so that the materials can be added rapidly and at the correct temperature.
Solution I. 38 grams of pure aluminum sulfate in 60 cc. water.
Solution II. 52.5 grams of crystalline sodium thiosulfate in 50 cc. water.
Solution III. 57 grams of sodium bichromate in 90 cc. solution.
Solution IV. 20 grams of dimethylaniline in 27 grams of concentrated hydrochloric acid.
Solution V. 25 grams of very finely ground manganese dioxide slurried with 30 cc. water.
The clear, neutral solution of p-aminodimethylaniline is made acid by the addition of 4 grams of concentrated sulfuric acid, and 100 grams of a 50 per cent, nonreducing solution of zinc chloride is added.
The beaker is placed on a felt pad, and a tube is arranged for blowing in steam. With thorough stirring, Solution I is added at room temperature, followed by Solution II, and 2 seconds later by one-third of Solution III (equivalent to 19 grams of sodium bichromate). The temperature of the mixture is raised to 40°C. in the course of 1 minute by the introduction of dry steam, and then Solution IV is added followed by the remainder of Solution III. The mixture is then heated rapidly to 70°. The solution becomes dark greenish blue in color due to the formation of the thiosulfonic acid of Bindschedler green. When the temperature reaches 70°, the slurry (V) is added and the temperature is raised to 85°.
The manganese dioxide is added to convert the sulfurous acid formed in the ring closure reaction to dithionate which is harmless. Equally good results can be obtained by using 40 grams of copper sulfate which is converted in the reaction to insoluble СигО.
The solution at 85° has a lustrous bronzy appearance as the dye precipitates from the concentrated zinc chloride solution. After 30 minutes, the mixture is allowed to cool to 50°C., and 70 grams of concentrated sulfuric acid is added to dissolve the manganese salts, aluminum hydroxide, and chromium oxide. The dye is filtered off at 20° and washed with a small volume of 10 per cent salt solution. This crude product is dissolved in 1 liter water at 100°, the solution is filtered, and the dye is reprecipitated by the addition of 50 grams of ordinary 50 per cent zinc chloride solution and 150 grams of salt. The zinc chloride double salt of the dye separates completely in 24 hours in the form of beautiful bronzy red crystals which are filtered off, washed with 10 per cent salt solution, and dried at 50° (no higher). The yield of pure, concentrated dye is about 44 grams.
The method described above was worked out by Bernthsen and Ulrich, who ;ilso recommended the use of aluminum thiosulfate. The practice of adding manganese dioxide or copper sulfate is generally followed. Relatively small quantities of dye are prepared in one run because rapid heating is important. The final dye is filtered off, using filters such as the one shown in Figure 27, page 151, and then placed in small bags and centrifuged.
Methylene blue is highly valued because it gives pure shades and is inexpensive. It is widely used for coloring tanned cotton. The zinc-free dye is used for discharging on silk. To produce the zinc-free dye, ordinary methylene blue is dissolved in water, and the zinc is precipitated by adding soda, leaving the easily soluble dye base in solution. The base is then precipitated from the filtered solution by adding salt. In large scale preparations, the crystallization requires several days, and is promoted by cooling, using lead pipes through which cold water is circulated.
Methylene green, the nitro derivative of methylene blue, is an interesting dye. The nitration is effected in the same way as that of tropaeo — line, using the crude zinc chloride double salt without further treatment.
The moist, crude methylene blue, as obtained in the above preparation, is mixed with 50 cc. water and 20 grams of 62 per cent nitric acid (40° Be); and 5 grams of sodium nitrite, dissolved in the minimum amount of water, is added at 25°C. The temperature is then raised to 50° with continuous stirring, and held at this point for 2 hours. The mixture is then diluted with 200 grams of saturated salt solution and filtered after 12 hours. The crude dye is redissolved in 1 liter water at 60° (not higher), the solution is filtered, and the dye is reprecipitated by the addition of 150 grams of salt and 50 grams of 50 per cent zinc chloride solution. The product is filtered off after 12 hours and dried at 45° until it can just be powdered. At this point, the dye still contains about 20 per cent of water, but it cannot be dried further without causing the color strength to decrease and part of the product to become insoluble. The yield is about 38 grains of concentrated material.
Methylene blue and methylene green are diluted with dextrin since the use of salts greatly decreases their solubility. Methylene green is used chiefly for producing black shades on silk, in combination with logwood-iron mordants and also with tin phosphate. The shades produced in this way are the most brilliant and fast blacks for silks.
When diethylaniline is used in place of dimethylaniline, the pure greenish thiazine blue is formed. (Monoethyl-o-toluidine gives tnionine blue.) Thiazine blue gives very pure blue shades on silk, but its importance is reduced by the availability of more fast alizarin dyes. The nonalkylatea methylene blue, diaminophenazthio — nium chloride or Lauth violet, is used in moderate amounts for producing pure violet shades. It is still prepared by the old method involving simultaneous oxidation of p-phenylenediamine and hydrogen sulfide with iron chloride.
 14 декабря, 2015
14 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 