In a 300-cc. flask, a mixture of 36.3 grams (0.3 mole) of dimethyl — aniline, 24 grams (0.2 mole) of 30 per cent hydrochloric acid, and 10.6 grams (0.1 mole) of benzaldehyde is heated for 12 hours under reflux. The end of the condenser should be closed with a plug of cotton or glass wool to prevent excessive oxidation of the aldehyde. Vigorous stirring throughout the reaction is necessary. At the end of 12 hours, the benzaldehyde has almost completely disappeared. The mixture is treated with 12 grams of soda ash, and the excess dimethylaniline is driven off. It can easily be recovered. The leuco base remaining behind is separated from
!l~* The schematic formula given for malachite green is that proposed by Fierz and Koechlin, Helv. Chim. Acta, 1, 210 (1918).
the water, pulverized, and washed once. The yield of dry product is about 24 grams.
(b) Oxidation of the Leuco Base to the Dye
(CHS), (CH,),
N N
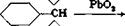 >—C—OH
>—C—OH
N N
(СН8)г (CH3)2
0.05 mole (16.5 grams) of the pure leuco base is dissolved in 300 cc. water and 20 grams of concentrated hydrochloric acid, and the solution is brought to 400 cc. and 0°C. by the addition of ice. To the well stirred solution is added, in one portion, a paste of lead peroxide made. from exactly 0.05 mole (16.5 grams) of lead nitrate (see page 138). After 2 hours, a solution of 25 grams of crystalline sodium sulfate is added to precipitate the lead as the insoluble sulfate which is filtered off. The dye is then precipitated with about 15 grams of soda ash and filtered off. It usually precipitates as a tarry material which, after drying, weighs about 16 grams (100 per cent of the theoretical amount).
Recrystallization of malachite green is not easily accomplished in the laboratory, large amounts being necessary for obtaining good results. The base (120 grams) is dissolved in a hot solution of 72 grams of crystalline oxalic acid in 300 grams of distilled water, and the boiling solution is filtered to remove any insoluble impurities. To the hot filtrate is added 7 grams of ammonium oxalate, in as concentrated a solution as possible, and the mixture is allowed to stand undisturbed. Best results are obtained by placing the solution in a large bath of hot water so that it cools slowly. In the course of a day, the temperature is lowered to 70°C., and the well formed crystals are then filtered off. The mother liquor yields a further quantity of impure dye on cooling (malachite green II). The yield of the oxalate is about 1.45 parts from 1 part of the base.
Technical Observations. Malachite green is still used in large quantities for coloring tin-weighted silk, wool, and paper. Pure compound shades are obtained inexpensively by using it in mixture with other dyes, but the tints are of only moderate fastness. The dye is also used in silk and cotton printing, but does not fulfill the modern demands for fastness properties so its use in these fields is declining.
The condensation is now carried out only with mineral acids, the older zinc chloride procedures having long since been abandoned. The Doebner process, starting with benzotrichloride, is also no longer used. The reaction can be effected with either hydrochloric or sulfuric acid. Hydrochloric acid gives the more rapid condensation, but requires the use of enameled apparatus whereas the sulfuric acid condensation can be carried out in leaded containers. It is important that excessive amounts of acid are not used, since under these conditions, a side reaction occurs leading to the formation of p-dimethylaminobenzohydrol which, of course, cannot give the dye.
In commercial preparations, several fractions of the dye are always separated since different consumers have quite different requirements as to the appearance of the product. The oxalate of malachite green corresponds to the formula, 2 C03H24N2 + З С2Н1Ю4. The crystallization requires several days and frequently crystals of great beauty are obtained. The addition of ammonium oxalate to promote crystallization is reminiscent of similar procedures in alkaloid chemistry and was discovered purely empirically.
Condensation of benzaldehyde with ethylbenzylaniline, sulfonation of the resulting leuco base with oleum, and oxidation of the leuco sulfonic acid with lead peroxide, produces light green SF (yellowish). The corresponding dye from o-chloro- benzaldehyde is erioviridine B. Both dyes have poor light fastness but are still widely used because of their color strength.
 5 декабря, 2015
5 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 