l — Aminonaphthalene-3,8-disulfonic Acid
To 900 grams of 100 per cent sulfuric acid, cooled to 5°C., is added, over a period of 15 minutes with good stirring, 384 grams of pure powdered naphthalene. Stirring is continued for 30 minutes and then 900 grams of oleum (64 per cent S03) is added slowly, not allowing the temperature to rise above 30°. When all of the oleum has been added, the mixture is warmed slowly to 40° and stirring is continued for 8 hours at this temperature.
10 grams of water is added, and then 305 grams of 62 per cent nitric acid (40° Be) is added dropwise. The nitration temperature is 15-20°. The mixture is allowed to stand overnight and is then poured into 3.5 liters of water.
|
e acid Several methods can be used for working up the reaction mixture. The mixture can be limed and reduced by the Bcchamp method (pages 75 and 77), or the nitro acids can be salted out with 1250 grams of salt. Another method consists in precipitating the 2-nitronaphthalene-4,8-disulfonic acid with 200 grams of ferrous sulfate, filtering it off, ana liming the mother liquor. Subsequent steps resemble those for Cleve acids, except that no magnesia is used. If the 2-nitronaphthalene- |
![]()
4,8-disulfonic acid is precipitated with ferrous sulfate, the precipitated acid and the limed mother liquor are reduced by the Bechamp method. The resulting reduced solutions are then evaporated to the point where they contain 20 per cent solids.
The l-aminonaphthalene-4,8-disulfonic acid is precipitated as the neutral sodium salt by the addition of enough salt to make a 20 per cent salt solution (30° Вё). The sulfonate is then filtered off. The l-aminonaphthalene-3,8-disulfonic acid is precipitated with hydrochloric acid as the acid sodium salt. If the whole mixture was limed, then the mother liquor is neutralized and evaporated to the point where salt separates. The supernatant liquid is poured off and allowed to crystallize at 20°C. The neutral sodium salt of 2-aminonaphthalene-4,8-disulfonic acid separates. This neutral salt is dissolved in water (24° Be solution) and precipitated by making the solution acid to Congo red with concentrated hydrochloric acid. The mixture is then allowed to cool, and the product is filtered off.
Depending on the method used, approximately the following yields are obtained: total yield, 55 to 68 per cent of the theoretical amount; of this, about 70 per cent is the 1,4,8 compound, about 16 per cent the 1,3,8 compound, and 14 per cent the 2,4,8 compound. In addition, other isomers are formed, such as the 1,4,7 and 2,4,7 compounds. These are, however, difficult to separate from the 2,4,8 isomer.*7
It is remarkable that yields higher than about 70 per cent of product titratable with nitrite are never obtained even in the most carefully executed preparations, even though all of the nitric acid is used (nitrometer determination). The reason must be that, in this case as well as in others, some of the amine is destroyed in the reduction. As in the preparation of H acid and the other naphthylaminesulfonic acids, it is always necessary to destroy the residual nitric acid before the reduction (see page 185).
The above sulfonic acids are important starting materials for preparing azo and other dyes (wool fast blue BL).*8
l-Naphthylamine-4,8-disulfonic acid can be converted by alkali fusion into 1-amino-8-naphthol-4-sulfonic acid (S acid). It can also be sulfonated further and the resulting sultam converted to the valuable Chicago acid. l-Naphthylamine-3,8> disulfonic acid can be converted into l-naphthol-3,8-disulfonic acid by heating the acid sodium salt with water to 180-200°C. Finally, 2-naphthylamine-4,8-disulfonic acid is used in preparing direct dyes, e. g., naphthogene blue RR.®9 The following reactions, and Table XI, show these various transformations.
HOsS NHS OH NH2
Iі ii
 NaOH 50% /s’ /іЧ
NaOH 50% /s’ /іЧ
*75° * Уу
SOjH SOsH
l-Amino-8- naph-
thol-4-sulfonic acid
(S acid)
87 Edelmann, Dissertation, Zurich, 1925.
08 Fierz-David, Kunstliche organische Farbstoffe, Springer, Berlin, 1926, p. 332.
60 Fierz-David, Kiinstliche organische Farbstoffe, Springer, Berlin, 1926, p. 160.
20,
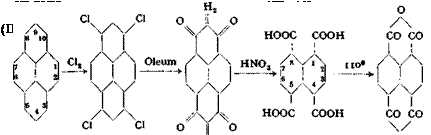 |
Naphthalene-1,4,5,8-tetracarboxylic Acid from Pyrene
Pyrene 3,5,8,10-Tetra — Di-peri — Naphthalene- Naphthalene-
сЫогоругепе naphthindane- tetracarboxylic tetracarboxylic — dione acid acid dianhyaride
 30 октября, 2015
30 октября, 2015  Pokraskin
Pokraskin 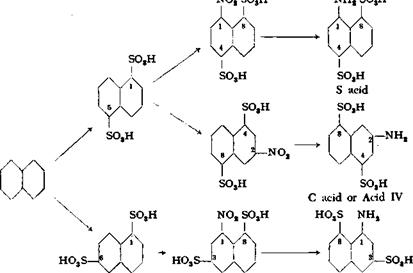
 Опубликовано в рубрике
Опубликовано в рубрике 