Alkali fusion of l-naphthylamine-3,6,8-trisulfonic acid yields H acid, the most important member of this series of intermediates in the dye industry. The preparation of H acid is a typical example of fusion reactions.
In order to obtain H acid in good yields, the temperature of fusion must not exceed 190°C. and the caustic soda must be at least 30 per cent. The following proportions are recommended for a laboratory preparation:
Naphthylaminetrisulfonic acid…. weight corresponding to
28 g. nitrite, or about 280 g. moist press cake
Caustic soda…………………………………………………………………… 130 g.
Water…………………………………………………………………………….. 130 g.
The fusion is carried out in an autoclave, an apparatus of such great importance both for the laboratory and for plant operations that a separate chapter has been devoted to its use (page 350). The ingredients listed above are placed in the autoclave and held, with continuous stirring, at 178-180°C. for 8 hours. The pressure is about 7 atmospheres.
The autoclave is then cooled and any excess pressure is released through the valve before loosening the top. If the fusion has been carried out properly, the fusion mixture is a dull, dirty yellow in color. If the color is too light, the fusion was not continued long enough. Too long a fusion, on the other hand, produces a mixture which is brown in color and which smells strongly of ammonia. Some ammonia is always formed, however, even under the correct conditions.
The reaction mixture at this point is a sirupy mass containing granular crystals of sodium sulfite. It is transferred to a 2-liter earthenware crock and diluted with 1 liter of water, and enough 50 per cent sulfuric acid is added to give the solution a definite and permanent acid reaction to Congo red. One must not be deceived by the free sulfurous acid set free in the reaction, since this volatilizes rapidly (hood!). The amino- naphtholdisulfonic acid precipitates as the acid sodium salt which is very insoluble in concentrated sodium sulfate solutions.* In spite of the low solubility of the product, the mixture should be allowed to stand several hours so that precipitation is complete. The product is then filtered off and washed with 10 per cent salt solution containing 1 per cent of hydrochloric acid. Excessive washing must be avoided to prevent loss of H acid. The material is finally pressed out in the screw press and dried at 100°. The yield is about 110 grams of 86 per cent H acid, corresponding to about 100 grams of 100 per cent material.
Concerning the analysis of H acid, see page 389.
18.
 |
l-Naphthylarnine-5- and -8-sulfonic Acids
The preparations of these acids closely resembles the preparation
* Solubility of H acid:
In water at 18°…………………………………………. 0.93%
In 10% NaCl at 18°…………………………………… 0.053%
In 10% NaCl + 0.8% HC1 at 18° . . . 0.023% In water at 100° 8%
of Cleve acids. The two acids are among the most widely used intermediates.
The most favorable conditions for preparing naphthalene-a-sulfonic acid involve sulfonating at a temperature (below 80°C.) lower than the melting point of naphthalene. To 260 grams of 100 per cent sulfuric acid, cooled to 0°, 128 grams of finely powdered naphthalene is added rapidly. (96 per cent of the powdered material should pass through a sieve having 400 openings per square centimeter. See page 175.) Sulfonation commences at once, and unless steps are taken to prevent it, the mixture will set up suddenly to a very hard mass when the crystals of naph — thalenesulfonic acid separate. When this happens, the stirrer is incapable of continuing. This occurrence can be prevented by seeding the mixture with a small amount of the solid sulfonic acid as soon as the introduction of the naphthalene has been completed. The seed crystals can be prepared by warming a small amount of naphthalene with sulfuric acid on a water bath and then cooling the mixture. The seed crystals induce rapid crystallization of the sulfonic acid as fast as it is formed and prevent its crystallization from a supersaturated solution. The sulfonation mixture thickens more slowly, and a sudden solidification does not occur.
The temperature seldom rises above 35°C., and it is necessary to use pure 100 per cent sulfuric acid or part of the naphthalene is not attacked. This is frequently the case in any event, so the mixture is warmed on a water bath to 60° with continuous stirring until all of the naphthalene has disappeared. It is difficult, without considerable practice, to effect smooth sulfonation on a small scale, although no undue difficulties are encountered in large scale sulfonations. To test for the presence of residual naphthalene in the reaction mixture, a small sample is diluted with water. Any unsulfonated naphthalene separates immediately.
The nitration reaction is carried out in exactly the same way as in the preparation of Cleve acid, except that here the second addition of sulfuric acid is unnecessary since it was all added at the beginning. The reduction step and isolation of the two isomeric naphthylaminesulfonic acids are also carried out as described for Cleve acids. The sodium salt of the 1,8 acid is, however, still less soluble than that of the 1,7 acid, and the isolation is therefore easier. The 1,8 acid obtained is practically free from Cleve acids, although a small amount is always present despite the low sulfonation temperature used. The yield of 1,8 acid is equivalent to about 100 grams of 100 per cent material; that of the 1,5 acid, obtained by precipitation with sulfuric acid, is equivalent to about 40 grams of 100 per cent material.
Variation: l-Naphthylamine-5- and -8-sulfonic acids — differ from the other sulfonic acids in that they can be isolated in the presence of large amounts of iron salts. Instead of neutralizing with lime and reducing the magnesium salt, the nitro acid, diluted with water, can be reduced directly with iron turnings. A necessary condition, however, is that the solution must be kept neutral to Congo red at all times. The temperature rises to about 80°C., and none of the sulfonic acid separates out. The mixture is heated to boiling for a considerable period, the violet coloration gradually changing to a greenish one. Boiling is continued while 40 grams of iron powder is added gradually, and the ferrous salts of the 1,5 and 1,8 acids separate as grayish white crystals. These salts are decomposed, after cooling the mixture, by the addition of sulfuric acid to make the solution definitely acid. The free sulfonic acids are then filtered off, washed thoroughly to remove iron sulfate, and the residue is dissolved in 1 liter of water containing 40 grams of magnesium carbonate. The solution is filtered and salt is added to make the solution 4 per cent with respect to salt. The 1,8 acid is precipitated as the sodium salt in extremely pure form, completely free from Cleve acids. After filtering off the sodium salt of the 1,8 acid, the filtrate gives the pure 1,5 acid on acidification. In this process, the Cleve acids are reduced to the hydroxylamine stage and then rearranged to the 1,4- aminonaphtholsulfonic acids which are washed out along with the iron sulfate.
Technical Observations. In large scale operations, the sulfonation of powdered naphthalene must be carried out somewhat differently than in the laboratory. In the first place, it is essential that the naphthalene be freshly ground because it soon reagglomerates. The best practice is to grind the material once on the day before it is to be used, and then again immediately before use, or if necessary, twice more. The addition of naphthalene to the sulfuric acid must be made as rapidly as possible. A good practical method of making this addition is to put all of the naphthalene into an open box and rake it from this into the opening in the sulfonation vessel. The material should be passed through a coarse sieve with a funnel-shaped bottom to prevent any lumps from getting into the reaction mixture. When all of the naphthalene has been added, the mixture is seeded and stirred up well with an iron spatula. An anchor-type stirrer is used, similar to the one shown in Figure 31. Even with very efficient cooling, the temperature of the pasty mixture rises slowly to about 18°C. and then suddenly to about 58° due to the heat of crystallization. Since these sulfonation conditions are more vigorous than in the laboratory preparation, the sulfuric acid should be diluted with ice to about 98 per cent. The sulfonation is completed in about 1.5 hours if the naphthalene was powdered finely enough.
If the alternate procedure is used, an iron reduction vessel cannot be used of course. For this process, wooden vats are used, and they last a long time.
The free sulfonic acids, after precipitation with sulfuric acid, are filtered off in filter presses having felt filters. The better the precipitate is washed, the easier will be the extraction with magnesia. It is advisable to wash the residue from the filtration at least twice with hot water since the magnesia-iron sludge retains appreciable quantities of the sulfonic acids.
The 1,8 acid cannot be precipitated in large scale operations by the addition of solid salt. Instead, a salt solution is added over a period of an hour, in order to prevent carrying down part of the 1,5 acid.
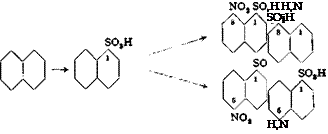
l-Naphthylamine-8-sulfonic acid is not used directly in making dyes, but only after conversion to other compounds, the most important of which are: naphthsul — tone, l-phenylaminonaphthalene-8-sulfonic acid, l-amino-8-naphthol-4-sulfonic acid, and l-amino-8-naphthol-2,4-disulfonic acid. The preparations of these compounds is shown in the following reactions:
![]()
|
1-Phenylamine- 8-sulfonic _ ____ HOjS NH2 SOjj NH |
|
so„H |
|
so, H |
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

І-Ашіпо-8-
naphtliol-4-sul-
fonic acid
(S acid)
1-Amino-8- naphtliol-2,4- disulfonic acid (Chicago acid or S. S. acid)
25°C. When the diazonium compound is heated to 55° in water solution, the naphthsultone (I) is formed in quantitative yield. The amount of the sultone formed is a direct measure of the purity of the starting material. The naphthsultone is almost always converted to the naphthsultonesulfonic acid, which yields very pure and light-fast dyes with diazo compounds. These dyes have decreased greatly in importance in recent years.
1 — Naphthylamine-в-sulfonic acid can also be converted to arylated products. Thus, the free sulfonic acid, when heated with aniline and aniline hydrochloride, yields the technically important l-phenylaminonaphthalene-8-sulfonic acid (II). One part of the pure, free sulfonic acid, mixed with three parts of aniline (or p-toluidine) and aniline (or p-toluidine) hydrochloride, is heated to 160°C. in an enameled kettle in an oil bath. The water which is always present is removed by vacuum distillation, and the heating is continued for 24 hours with stirring. The excess aniline is then removed by careful vacuum distillation, after which the aniline salt of the phenylated acid is decomposed by the addition of the calculated amount of hydroxide solution, and the remaining aniline is removed by steam distillation. The resulting solution, containing the desired phenylaminonaphthalenesulfonic acid, is coupled directly with H acid in acetic acid, without first isolating the phenylated sulfonic acid. The dye prepared in this way is sulphon acid blue R, which is fast to light.
Sulfonation of l-naphthylamine-8-sulfonic acid with oleum gives, depending on the conditions, either the di — or trisulfonic acid of naphthylamine (as well as the naphthsultamdisulfonic acid8®). Alkali fusion of these compounds yields the corresponding aminonaphtholsulfonic acids (III and IV), which are starting materials for wool and cotton dyes.
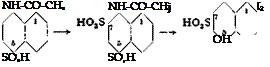 |
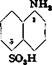 |
l-Naphthylamine-5-sulfonic acid is less important and deserves but little comment. It is either diazotized and coupled with amines and naphthols, or converted to l-amino~5-naphthol-7-sulfonic acid (M acid). As shown by the reactions:
it is necessary to acetylate the naphthylaminesulfonic acid first (in contrast to the 1,8 isomer), because it would otherwise be destroyed by the sulfuric anhydride. Such acetylations are rather widely used in the dye industry (see amidonaphthol red G).
60 Wielopolski, Dissertation, Zurich-Linz, 1936.
 29 октября, 2015
29 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 