|
p Salt and NaCl (grams in 100 grams of solution) |
|||||||||
|
25 |
°С. |
30°c. |
40°C. |
50°C. |
65′ |
■c. |
|||
|
p Salt |
NaCl |
p Salt |
Nad |
P Salt |
NaCl |
p Salt |
Nad |
p Salt |
NaCl |
|
5.58 |
0 |
6.24 |
0 |
7.98 |
0. |
9.75 |
0 |
14.6 |
0 |
|
3.46 |
2.38 |
1.21 |
4.84 |
1.46 |
5.62 |
4.15 |
2.9 |
8.47 |
2.93 |
|
0.31 |
9.19 |
0.16 |
13.08 |
0.65 |
8.47 |
2.17 |
5.42 |
6.12 |
3.81 |
|
0.15 |
13.16 |
0 |
26.5 |
0 |
26.70 |
1.05 |
8.39 |
1.96 |
7.19 |
|
0 |
16.81 |
0 |
26.8 |
1.26 |
10.83 |
||||
|
0 |
26.43 |
0 |
272 |
||||||
|
p Salt and NaaSOt (grams |
in 100 grams of solution) |
||||||||
|
25°C. |
30°C. |
40°C. |
50°C. |
65°C. |
|||||
|
P Salt |
Na2SOi |
p Salt |
Na2SO, |
p Salt |
Na2S04 |
p Salt |
NajjSO* |
p Salt |
Na2S04 |
|
3.42 |
1.97 |
1.97 |
4.81 |
4.3 |
2.85 |
5.72 |
2.87 |
11.75 |
1.68 |
|
2.41 |
3.06 |
0.26 |
13.23 |
2.18 |
5.83 |
3.49 |
5.35 |
7.37 |
5.28 |
|
1.78 |
4.34 |
0 |
29.1 |
1.2 |
8.48 |
1.93 |
8.24 |
6.7 |
5.45 |
|
0.93 |
7.4 |
0.77 |
10.92 |
1.42 |
10.01 |
1.90 |
12.0 |
||
|
0.62 |
9.25 |
0 |
32.5 |
0 |
31.9 |
3.14 |
10.86 |
||
|
0.52 |
10.52 |
0.25 |
26.96 |
||||||
|
0.10 |
13.15 |
0 |
31.0 |
||||||
|
0 |
21.9 |
sodium naphtholate is formed. The watery consistency of the mixture
permits the addition of much more sodium sulfonate than most pro-
cedures specify. In laboratory preparations, 1.5 parts of the sulfonate
(300 grams in this case) to 1 part of alkali can easily be added. (In
plant processes using correctly designed equipment and proper firing,
2.8 parts of the salt can be used for each part of alkali, and charring or excessive thickening of the mixture is not encountered.) When a temperature of 290° has been reached, about one-half of the sulfonate should have been added. The temperature is then raised carefully to 300° and, when three-fourths of the salt (225 grams) has been added,
to 305°. Finally, the temperature is raised to 318° when all of the salt has been added. Higher temperatures should not be used. The melt becomes gritty due to the sodium sulfite which has separated, and the naphtholate gradually replaces the sulfonate, which disappears slowly. The melt is held at 318° for 15 minutes, carefully avoiding overheating. The whole operation requires about 1 hour. Too rapid addition of the sulfonate results in charring and lowering of the yield.
The reaction mixture is poured out into a low dish, and after cooling, it is broken up and returned to the fusion kettle with 500 cc. water. Most of the mixture dissolves easily on careful warming, leaving a crust of undissolved sodium sulfite. The solution is poured off and more water is added until all the material is dissolved. More than 2 liters water should not be required. The combined solutions are placed in a porcelain dish and heated to boiling over a Fletcher burner. Sulfuric acid (50 per cent) is added until the reaction to thiazole paper almost disappears. The solution is then cooled slightly and filtered through a large suction funnel into a warm flask. The volume of neutralized solution ready for filtration is about 3 liters. It is colorless or light yellow.
The clarified solution is heated to boiling and enough 50 per cent sulfuric acid is added to make the solution strongly acid to litmus. (If there is not time to filter off the /J-naphthol within an hour’s time, hydrochloric acid should be used. Otherwise, sodium sulfate is also precipitated.) There is no odor of sulfurous acid. The /З-naphthol is insoluble in neutral sulfite solution in the presence of some bisulfite. It separates as an oil at first, but solidifies immediately. After an hour, the precipitate can be filtered off without losing more than traces. The product is collected on a suction funnel with a cotton filter, washed thoroughly with cold water, and dried at low temperature, either in a vacuum drying oven or in an ordinary drying oven. If the drying temperature is too high, the product melts and sublimes.
The yield of dry, crude naphthol from 300 grams of /? salt is about 150 grams (93 per cent pure), of distilled product about 135 grams, m. p. 122°.
The crude product suffices for some uses, but it must be thoroughly purified to meet the specifications for the commercial product. For this purification, only vacuum distillation is used today.
Technical Observations. The sulfonation of naphthalene is carried out in huge cast iron kettles holding 1000 to 3000 liters. Heating is done either directly with generator gas or by means of a steam jacket (double wall) (Figure 31) which must withstand at least 6 atmospheres in order that the required temperature of 174° can be attained.
Instead of converting the a-sulfonic acid into the p isomer by long heating, another scheme is used in the industry. After several hours of heating, steam is blown into the sulfonation mixture, whereby the a acid is split into naphthalene and sulfuric acid, leaving the p acid unchanged. In this way, a mixture of the p acid and sulfuric acid is obtained, substantially free from the isomeric sulfonic acid. The technical process is different from the laboratory procedure in other respects also. Instead of using salt, which is much too costly, to precipitate the
|
Fig. 31. Sulfonation and nitration kettle with steam jacket (double wall). |
naphthalene-^sulfonic acid, the sulfonation mixture is treated with the waste liquor from the naphthol, which contains sulfite. Sulfur dioxide is given off and is passed into the diluted 0-naphthol melt. The 0-naphthol is thereby precipitated, and, as pointed out, the resulting mother liquor serves in precipitating the p — naphthalenesulfonate. Only in this way can the 0-naphthol be manufactured cheaply. Abo, careful extraction of the crude p-naphthol with dilute sodium hydroxide recovers the a-naphthol present, since the latter is more easily soluble in alkali. The determination of mixtures of a — and ^-naphthol is described in the Analytical Section. In some ^-naphthol plants, the washed crude naphthol is fused in closed iron kettles (the aqueous mother liquor is drained off) and dried in vacuum. It is then filtered hot through a small filter press. In this way, most of the salts present in the crude naphthol are retained in the filter press, and the subsequent vacuum distillation goes very smoothly with little pitch formation. Technical ^-naphthol is up to 99.9 per cent pure. It has been marketed in recent years in the flake form which is much easier to work with and is free from dust.
 18 октября, 2015
18 октября, 2015  Pokraskin
Pokraskin 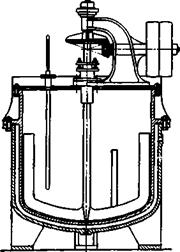
 Опубликовано в рубрике
Опубликовано в рубрике 