(a) Phenylglycine-o-carboxylic Acid"
COONa л COOH
/V
I + C1CH,—COONa = + NaC1
NH—CH,—COONa
A paste of 137 grams (1.0 mole) of anthranilic acid (page 174) in a small amount of water is exactly neutralized with approximately 120 grams of 40° Вё sodium hydroxide solution. A second solution is prepared by dissolving 94.5 grams (1.0 mole) of chloroacetic acid in 200 cc. water and exadtly neutralizing it by the addition, with stirring, of about 55 grams of soda ash. The two solutions are mixed and held at 40°C. for 4 days. The monosodium salt of phenylglycine-o-carboxylic acid which crystallizes out is filtered off with suction, washed with a small amount of cold water, and dried to constant weight in a steam heated oven. The yield is about 75 per cent of the theoretical amount.
(Ь) Indoxylcarboxylic Acid
A mixture of 25 grams of sodium hydroxide (completely dehydrated by fusing in an iron crucible), 25 grams of potassium hydroxide (similarly dehydrated), 7.5 grams of caustic lime (dehydrated by igniting without sintering in a porcelain crucible), and 25 grams of thoroughly iried monosodium phenylglycine-o-carboxylate is ground in a ball mill with exclusion of moisture. The resulting intimate mixture is heated in a vacuum baking apparatus (Fig. 30, page 181) in a graphite bath for 2 hours at 150°C., then for 6 hours at 230-235°. A homogeneous, yellow brown, hard mass is formed.
(c)
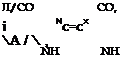 |
|||
Indigo
The fusion mixture from (b) is dissolved in 2 liters water at 80°C., and a vigorous stream of air is passed through the solution until no more indigo is formed in a filtered test sample when shaken with air. The precipitated dye is filtered off, washed with water, boiled with dilute hydrochloric acid to remove all of the lime, filtered again, washed thoroughly with water, and finally dried in a steam heated oven. The yield is 12 to 12.5 grams, or 80 to 82 per cent of the theoretical amount based on the phenylglycine-o-carboxylic acid.
The foregoing procedure, discovered by Heumann and worked out by the Badische A. S.F., was the first process used on a large scale for the preparation of indigo. It was kept in operation for a long time, along with the related process (Deutsche Gold- und Silberscheideanstalt) employing potassium and sodium hydroxides in combination with sodium amide, and only recently has it been replaced by other processes.
 16 декабря, 2015
16 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 