In a glass vessel equipped with reflux condenser and stirrer (Fig. 6), a solution of 20 grams of gallamide (page 170) of about 92 per cent purity (the purity is determined by distilling off the ammonia from a sodium hydroxide solution and titrating) in 500 cc. 90 per cent alcohol is heated to boiling, and the nitrosodimethylaniline hydrochloride, prepared from 75 grams of dimethylaniline, is added in three portions, allowing a 15-minute interval between additions. The mixture is then refluxed for 4 hours and allowed to stand for 12 hours more. The gallamine blue comes out as a lustrous, bronzy precipitate which is filtered off and washed with water. The alcohol is recovered and rectified. The yield of pure gallamine blue is about 40 grams. The alcoholic mother liquor yields a gray, nigrosine-like dye which gives very fast gray tints on cotton (with chromium acetate); this dye is called methylene gray.
Gallamine blue, which is quite insoluble in water and hence cannot be used as such, can be converted to soluble forms in various ways.
One part of gallamine blue is heated to 50° C. on a water bath with 6 parts of sodium bisulfite solution (25 per cent S02) until the evolution of sulfur dioxide ceases (about 1 hour). The mixture is then heated for 1 to 3 days at 85° until the mixture has become gray green. The resulting product is the sulfonic acid of the leuco compound (perhaps complex sulfonate) which, with chromium acetate, gives brilliant and fast marine blue shades on wool. It can also be used in cotton printing, but is not as important for this use as the related dye, modern violet.
Reduction of gallamine blue with hydrogen sulfide produces a leuco compound, modern violet, which gives extremely pure and fast chromium lakes on cotton.
|
Modem violet |
To the clear solution of 50 grams of gallamine blue in about 40 grams of 30 per cent sodium hydroxide and 400 cc. water, 50 grams of crystalline sodium sulfide is added. During the course of 1 hour at 60°C., the mixture is slowly acidified by the addition of about 100 grams of concentrated hydrochloric acid until a permanent acid reaction to Congo red is produced. The blue color almost disappears, and the nearly colorless solution is filtered to remove sulfur. The filtrate is treated with 150 grams of salt to precipitate the leuco compound which is filtered off, washed with a small amount of saturated salt solution, and pressed out well. The product should be dried in vacuo at 60° since it is easily reoxidized. The yield is about 55 grams.
Technical Observations. Industrial preparations are carried out in enameled iron vessels with lead-tube reflux condensers. The preparation starting with 40 kilograms of gallamide requires about 12 hours.
Modern violet must be powdered in the cold because its high oxidizability may cause spontaneous ignition. This may be caused, in some cases, by the presence of finely divided sulfur.
The oxazines are excellent printing colors. In addition to dimethylaniline derivatives, those from diethylaniline are also prepared. The latter give very pure greenish tints. If gallic acid is used in place of gallamide, gallocyanine is formed. This dye was first discovered by H. Kochlin, who, in trying to fix nitrosodimethylaniline to cotton by means of tannin and tartar emetic, obtained blue dyes which he recognized as oxazines. Gallocyanine cannot be prepared satisfactorily in ethyl alcohol solution. Methyl alcohol gives better results, but is an undesirable solvent because of its toxicity and volatility. Many more complicated oxazines are known but these cannot be described here.
It should be mentioned in passing that the first oxazine to attain technical importance was Meldola blue (naphthol blue, Bengal blue), prepared from nitrosodimethylaniline hydrochloride and ^-naphthol. It is a very fast dye but does not give pure shades. Furthermore, its dust irritates the mucuous membranes so severely that many persons cannot work with it. Despite these disadvantages, however, — Meldola blue is still rather widely used.
 10 декабря, 2015
10 декабря, 2015  Pokraskin
Pokraskin 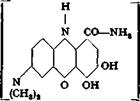
 Опубликовано в рубрике
Опубликовано в рубрике 