G acid is subjected to fusion with NaOH and a small amount of water at 200° in an open vessel to yield 2,8-dihydroxynaphthalene-6-sulfonic acid. This product
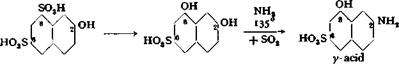 |
is not isolated, but is treated directly with ammonium sulfate, neutralizing part of the free alkali with sulfuric acid.
This process gives a very satisfactory product and has the advantage that pressures higher than 15 atmospheres are not required. It is noteworthy that the p-hydroxyl group, and not the а-group, is aminated (cf. page 182).
This process involves the same reactions used in the process above, but in reverse sequence; alkali fusion first, followed by the Bucherer reaction. In this case, aqueous ammonia is not used, but instead the inexpensive ammonium sulfate which is decomposed by the alkali in the fusion mixture to generate ammonia. Furthermore, the sulfite formed in the fusion reaction is enough to effect the Bucherer reaction.
 |
Phenyl-gamma Acid
A mixture of 239 grams of 100 per cent у acid, 750 cc. water, 750 grams of sodium bisulfite solution containing 25 per cent S02, and 200 grams of aniline is boiled under reflux for 24 hours. Concentrated soda solution is then added to make the solution distinctly alkaline, and the aniline is removed by steam distillation. On acidification with concentrated hydrochloric acid, the phenyl-у acid is precipitated. The yield is about 270 grams of 90 per cent product, or 75 to 80 per cent of the theoretical amount.
16, l-Naphthylamine-3,6-disulfonic Acid (Freund Acid)
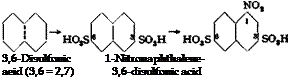
![]()
![]()
![]() J S03H
J S03H
In the apparatus described on page 101, 128 grams of pure naphthalene is heated to 165°C. with stirring. During the course of 15 min
utes, 400 grams of 100 per cent sulfuric acid is added, and the mixture is heated for 1 hour at 165°C. to complete the disulfonation. It is not advisable to heat above 165°, or as much as 30 per cent of the 2,6-di — sulfonic acid is formed and this gives a product quite similar to that from the 2,7 (3,6) isomer.
It is relatively simple to determine the extent to which a sulfonation reaction has proceeded. The reaction mixture is neutralized with barium carbonate and treated with enough soda to form the sodium spit. The number of sulfo groups present can be calculated from the amount of soda used up. One sulfo group requires 53 grams of ЫагСОз. Calcium carbonate cannot be used for this purpose since calcium sulfate is soluble in solutions of naphthalenesulfonic acids.
The reaction mixture is now cooled to 15°C., and 103 grams of 62 per cent nitric acid (40° Be) is added over a period of 1 hour, keeping the temperature below 30° since dinitro compounds are easily formed. The solution is held at room temperature for at least 10 hours and is then poured into 1 liter cold water with stirring. The resulting mixture is heated to 70°, and a stream of air is bubbled through it until no more nitric acid oxides are given off. The excess nitric acid is then destroyed by the addition of ferrous sulfate, exactly as was done in the preparation of Cleve acids (page 185), and 150 grams of anhydrous sodium sulfate (or the corresponding amount of the hydrated salt) is added to form the sulfonate. The succeeding steps of removing excess sulfuric acid by means of chalk (about 400 grams), filtering off the calcium sulfate, and reduction, are carried out in the same manner as in the Cleve acids preparation (page 185), with the single exception that here the iron is etched with hydrochloric acid (20 cc. concentrated acid) instead of with acetic acid.
The solution from the reduction is neutralized with soda (testing with ammonium sulfide to be sure that all of the iron has been precipitated ) and filtered, then evaporated to 800 cc. and treated hot with 100 grams of potassium chloride. When the latter has dissolved, the solution is acidified at 100° with 100 cc. 30 per cent hydrochloric acid and allowed to cool. The acid potassium salt separates over a period of 12 hours, the whole mass setting to a solid. The pasty mixture is filtered on a large suction funnel with a double filter paper, rinsing the residue into the funnel with the mother liquor and washing the precipitate with 200 cc. saturated salt solution. The product is pressed out as completely as possible in a screw press. The moist press cake weighs 300 to 320 grams, about 270 grams when dry, and corresponds to about 35 grams of sodium nitrite (see page 206).
The mother liquor is dark colored and uses about 22 grams of sodium nitrite, especially if the reduction has not been carried out properly. It contains hydroxyl —
amine compounds which give a violet solution on diazotization. The sodium salt can be prepared instead of the potassium salt, by using 100 grams of sodium chloride in the precipitation. The precipitate is much more difficult to filter, however, and the final product is therefore not quite so pure as when the potassium salt is used. By dissolving the press cake in four parts of boiling water and recrystallizing, Freund acid is obtained in a very pure state, which is especially suitable for use in the preparation of complicated polyazo dyes.
17.
l-Amino-8-naphthol-3,6-disulfoiiic Acid (H Acid)
HO, s
|
8 v 1 N / 8 V 1 il* |
![]()
HO, S NH,
(a) l-Naphthylamine-3,6,8-tri»ulfonic Acid (Koch Acid)
In an iron vessel (or a glass flask or porcelain beaker with a tight lead cover) of about 1-liter capacity, 128 grams of pure naphthalene is heated to 150°G., and 140 grams of 100 per cent sulfuric acid is added with good stirring, allowing the temperature to rise to 160-165°. The mixing is completed in 10 to 15 minutes and stirring is continued at 160-165° for 30 minutes more. The reaction mixture is then cooled to 100°, an additional 260 grams of 100 per cent sulfuric acid is added in one portion, and the whole is cooled further to 30°. While the temperature is held at this point, 400 grams of 60 per cent oleum is added in the course of 30 minutes, and the mixture is stirred at 25° for 3 hours, then for 7 hours at 165° to complete the trisulfonation (plus some unavoidable tetrasulfonation). The reaction vessel is now placed in ice water and the mixture is cooled to 10°, 15 grams of ice is added, and 103 grams of 62 per cent nitric acid (40° Be) is added dropwise. The temperature must not exceed 10° or extensive oxidation will occur. The nitric acid can be added in about 1 hour. The reaction mixture is allowed to stand overnight, and is then poured into 2 liters cold water. Nitric oxide is given off, and the temperature may rise to 70° without damage. Air is blown through the solution for about 1 hour at 70° to remove most of the nitric oxide, the last parts of which are removed by the addition of a 20 per cent solution of ferrous sulfate (test a diluted portion of the mixture with starch-iodide paper or sulfone reagent; see page 185).
The resulting sulfuric acid solution is cooled to 30°C., and to it is added, with continuous stirring, 180 grams of iron nails in small portions. The temperature rises to about 60° in the course of 2 hours. Stirring is continued for 4 horns, and then the temperature is increased to 70° over the course of the next hour. Part of the iron goes into solution. On standing overnight, the mixture precipitates the iron salt of
l — naphthylamine-3,6,8-trisulfonic acid (Koch acid). It is then heated to 90° whereupon everything but the unused iron goes into solution. The supernatant liquid is poured off, and the acid sodium salt of Koch acid is precipitated by the addition of solid salt with thorough stirring. Enough salt is used to make the solution 18 per cent with respect to sodium chloride. The ferrous sulfate is not precipitated. The mixture is allowed to cool to 25° and is filtered without delay (because of the danger of the iron salts crystallizing) through a triple filter on a large suction funnel. The precipitate is washed thoroughly with 20 per cent salt solution to remove all of the iron salts and sulfuric acid. It is then dissolved in 1 liter hot water containing the necessary amount of soda, and the solution is filtered. The filtrate is reheated to 80°, and the Koch acid is precipitated by the addition of salt (to make the solution 18 per cent salt) and enough hydrochloric acid to produce a strongly acid reaction to Congo red. The acid sodium salt of l-naphthylamine-3,6,8- trisulfonic acid separates as a snow-white precipitate. After 12 hours, it is filtered off and pressed out in a screw press. The yield is about 350 grams of moist press cake corresponding to about 38 to 42 grams of nitrite, or about 55 to 60 per cent of the theoretical amount.
Technical Observations. The manufacture of Koch acid is one of the most important operations in the whole field of dye chemistry, because from it are prepared two of the most widely used intermediates of the dye industry: H acid (1- amino-8-naphthol-3,6-disulfonic acid) and chromotropic acid (l,8^dihydroxynaph- thalene-3,6-disulfonic acid). In large scale preparations, 2 moles (256 kilograms) of naphthalene is usually sulfonated at one time with the addition of NaaSOi to ensure smooth reaction. Maintainance of the necessary temperature is rather difficult. Superheated steam is usually used for heating (Fig. 31) since this gives rapid heating, but does not prevent rapid cooling. The nitration step is especially difficult because the naphthalene-1,3,6-trisulfonic acid which is formed tends to separate, with the result that the rest of it, which remains dissolved, is easily oxidized by the nitric acid. To avoid this, the nitration is started at temperatures as high as 80°, which is much too high, of course, but does have the advantage that crystallization is prevented. After a small amount of nitric acid has been added, the mixture is cooled to 20° without risking separation of the trisulfonic acid. The other operations resemble closely those carried out in the laboratory, and no further remarks on them are necessary.
, All variations of the steps in this synthesis have been studied. A few of these variations might be mentioned here. Instead of using an acid reduction, the nitration mixture can be limed and then reduced in the presence of a small amount of acid exactly as in the case of Cleve acids (page 185). The nitrotrisulfonic acid can also be salted out easily and filtered off. This, however, offers difficulty in industrial work, since it involves working with strongly acid solutions. The sulfonation can be modified so that the intermediate product is largely naphthalene-2,7-disulfonic acid instead of the 1,6 isomer as in the procedure given here.
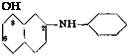
The 2,7-disulfonic acid can be used to prepare H acid by a different method, as shown in the following reactions:
|
|
This series of reactions gives about the same yield as the process described here, but the H acid obtained is not quite so pure.
 28 октября, 2015
28 октября, 2015  Pokraskin
Pokraskin 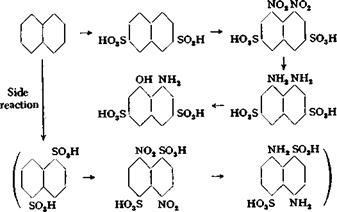
 Опубликовано в рубрике
Опубликовано в рубрике 