![]()
N—CHa—
In a glass flask equipped with a stirrer is placed 150 grams of 100 per cent sulfuric acid and to this is added carefully, over a period of 15 minutes, 150 grams of ethylbenzylaniline, maintaining the temperature of the mixture below 50° by means of external cooling. Then 150 grams of oleum (60 per cent SOs) is added slowly, and the mixture is held at 60° until a small test sample shows no turbidity when diluted with water and neutralized with dilute soda solution. This usually requires about 3 hours. The reaction mixture is then poured into 1 liter of water, and after 12 hours the free sulfonic acid which has separated is filtered off
42Blangey, Fierz, and Stamm, Helv. Chim. Acta, 25, 1162 (1942).
and washed with water, giving a pure fraction of about 72 per cent of the theoretical yield. The mother liquor, containing sulfuric acid, can be neutralized with lime, treated with sodium sulfate, filtered to remove CaS04, and evaporated to 400 cc. There is obtained — frequently only after scratching with a glass rod or seeding — a second portion of the same sulfonic acid, amounting to about 5 per cent of the theoretical amount. The second mother liquor yields the sodium salt of the isomeric para acid on treatment with salt (about 15 per cent yield).
Tetramethyl-p, p’-diaminodiphenylmethane from Dimethylaniline
and Formaldehyde
(CH,)aN-/ )> + CHaO + —N(CHa)t -►
![]()
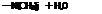
 (CH3)jN-
(CH3)jN-
A mixture of 242 grams (2 moles) of dimethylaniline, 90 grams (1.0 mole + 20 per cent excess) of 40 per cent aqueous formaldehyde, and about 1 gram of pure sulfanilic acid, is boiled under reflux with vigorous stirring. No visible change in the reaction mixture occurs, and after 8 hours a small sample of the light yellow emulsion is pipetted out and cooled. The oil should solidify completely, and on heating, the mixture should have only a faint odor of dimethylaniline. Otherwise the boiling under reflux should be continued for a longer time.
When the reaction is complete, the reaction mixture is steam distilled until no more dimethylaniline and formaldehyde come over. The distillate should contain only a few drops of dimethylaniline. The residue is poured into a large volume of cold water, whereupon the base solidifies immediately. The water is decanted and the solid lumps washed thoroughly several times with water to remove formaldehyde. The product is finally melted under water and allowed to solidify.
The base obtained in this way is a hard, yellowish white, crystalline mass with melting point between 80 and 90°C. One recrystallization from 500 cc. alcohol yields the pure base in nearly white, glistening crystals having the correct melting point of 91°. The yield of crude material is quantitative; that of recrystallized base is over 90 per cent of the theoretical amount.
Tetramethyldiaminodiphenylmethane (“methane base” in the industry) is the starting material for the preparation of auramine (q. v.). By oxidation with lead dioxide, it yields tetramethyldiaminobenzohydrol (see next preparation).
 28 сентября, 2015
28 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 