If G acid is desired as the chief product, the reaction is effected with a larger quantity of sulfuric acid and higher acid strength, at a moderate temperature, in no case over 60°C.
To 200 grams of 100 per cent sulfuric acid, well stirred and cooled in running water, is added 72 grams (0.5 mole) of pure, finely powdered /J-naphthol at such a rate that the temperature does not rise above 20°. The mixture is stirred at room temperature for 1 hour, after which a test sample should form a clear solution with water and the diluted solution should show no turbidity on heating. Again with cooling, 100 grams of 20 per cent oleum is added slowly, while the temperature is not allowed to rise appreciably above 20°. The reaction mixture is now heated in a water bath to 55-60° and stirred at this temperature for about 40 hours. At this point a test sample, diluted with water and made slightly alkaline with soda, is treated with diazotized p-aminoace — tanilide. Only the easily salted out, blue red, R salt dye should be formed, and none of the more soluble orange red dye from Schaeffer salt (see preceding section). Toward the end of the sulfonation, a crystalline precipitate usually separates, and the reaction mixture becomes a thick paste which is, however, easily stirred. The mixture is poured into 1 liter water, 75 grams of anhydrous sodium sulfate is added, and the solution is stirred for 1 hour at 95°, replacing the water lost by evaporation. By this treatment, any 2-naphthol-l,6-disulfonic acid and 2-naphthol-l,3,6-trisulfonic acid are converted to Schaeffer
acid and R acid, respectively. The hot solution is then neutralized by adding about 300 grams of chalk with stirring. This addition can be made rather rapidly without danger of foaming over. Filtering and washing of the calcium sulfate precipitate, removing the remaining calcium compounds by precipitation wtih soda ash (about 25 grams), and reacidifying the filtrate with concentrated hydrochloric acid (about 30 cc.) are carried out exactly as in the preparation of Schaeffer acid. Finally, the solution is evaporated to 400 cc., treated hot with 60 grams of salt, and allowed to cool with stirring. Most of the R salt, and some Schaeffer salt which is present, crystallize out on standing overnight. The precipitate is filtered off and washed with half-saturated salt solution, proceeding exactly as described in detail for the isolation of Schaeffer salt (page 194). The dried product weighs about 65 to 70 grams. Titration with diazotized p-aminoacetanilide (120 cc. 1N solution) and with diazotized p-nitroaniline (150 cc. 1 N solution) shows that the product corresponds to about 30 per cent of the /J-naphthol used and contains about 80 per cent R salt with a very small amount of Schaeffer salt, and 20 per cent G salt. Pure R salt can be obtained by the method described on page 196.
The filtrate is reheated to boiling, treated with 100 grams of potassium chloride, allowed to cool with stirring, and then left overnight. The precipitate is filtered off, washed thoroughly with three 50-cc. portions of 10 per cent potassium chloride solution, and dried in a steam heated oven. The product weighs 140 to 150 grams and corresponds to about 300 cc. 1 N solution of diazotized p-nitroaniline. Thus, the yield is about 60 per cent of the theoretical amount. In carefully conducted preparations, the colorless product is pure G salt which gives no immediate dye formation with diazobenzene in weakly alkaline, dilute solution.
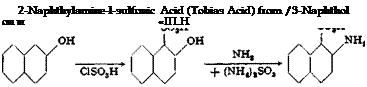 |
The mother liquor still contains about 10 per cent of the p-naphthol in a complex mixture with various sulfonic acids which cannot be separated in any way which is feasible technically.
 23 октября, 2015
23 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 