(a) Benzal Chloride
In a chlorination apparatus such as that shown in Figure 5 (page 63), 460 grams (5 moles) of toluene containing 10 grams of phosphorous pentachloride is heated to boiling, and chlorine is run in until the gain in weight is 345 grams.
Sunlight or ultraviolet light generally facilitates smooth chlorination of the side chain, especially in the case of chlorotoluenes (see page 162). A 600-1000 watt lamp can be used satisfactorily in the laboratory as the light source.
![Derivatives of Toluene Benzal Chloride and Benzaldehyde from Toluene Подпись: CH.C11 СНСЦ CC1,1 / л л 1 1 + ]+ Л/ - IV J](/img/1206/image166_0.gif) |
|
The chlorination requires about 6 hours. The resulting mixture of unchanged toluene, benzyl chloride, benzal chloride, and benzotri — chloride, is distilled through a glass-bead column, and the fraction boiling at 160-225°C. is collected separately. This fraction chiefly contains benzal chloride, boiling at 204°, along with small amounts of benzyl chloride and benzotrichloride. It is possible, particularly on a large scale, to separate the individual compounds by precise fractionation.
Benzoic acid
(b) Benzaldehyde
Benzal chloride, used for the preparation of benzaldehyde, should be free from benzyl chloride. It should be fractionated carefully, therefore, removing the portion boiling below 180°.
The benzal chloride (161 grams, 1.0 mole), which may contain some benzotrichloride, is placed in a small glass flask with 0.5 gram of iron powder and heated to 30° with stirring for 30 minutes. 25 grams of water is then added, and the mixture is heated carefully until, at about 100°, generation of hydrogen chloride begins. The reaction then proceeds spontaneously for a time, and is finally brought to completion by gentle heating. Soda is then added in an amount sufficient to make the mixture distinctly alkaline to litmus, and the benzaldehyde is steam distilled out of the reaction mixture. The residue in the distillation flask is filtered, made acid with hydrochloric acid, and cooled to obtain benzoic acid in pure white form. The distillate from the steam distillation contains products, along with the benzaldehyde, which cannot be removed completely by fractional distillation. Therefore, the distillate is treated with technical sodium bisulfite solution, and allowed to stand for some time, after which the oily residue is removed. Depending on the volume of the distillate, 230 to 350 grams of sodium bisulfite solution containing 25 per cent S02 is required. Soda or sodium hydroxide is added to the clear liquid to make it distinctly alkaline, and the benzaldehyde is separated in a separatory funnel and distilled at ordinary pressure. The yield of benzoic acid amounts to about 12 grams, that of benzaldehyde to about 80 grams (b. p. 178-179°).
Technical Observations: Toluene cannot be chlorinated in an iron vessel, as
benzene can, because, in the presence of iron, the chlorine goes into the ring. It is necessary, therefore, to use glass, enameled, or porcelain apparatus for this chlorination (see also dichlorobenzaldehyde, page 162). The addition of phosphorous penta — chloride can often be omitted, since it is not essential but has only an accelerating action. In large scale operations, the hydrolysis of benzal chloride is carried out in a copper apparatus and the separation of the benzaldehyde is made in large lead — lined separating funnels with glass level gauges. The method described above[34] has entirely replaced the older method starting with benzyl chloride and converting this to benzaldehyde by the action of water and lead nitrate.
There is, however, another process which favors, to a considerable degree, the formation of benzoic acid, a valuable product, and which is also used on a large scale. This method involves the oxidation of toluene in concentrated sulfuric acid by means of pyrolusite or manganite (see xylene blue VS).
Still another method consists in converting toluene to benzyl chloride, hydrolyzing this to benzyl alcohol with aqueous sodium carbonate at 120°, and oxidizing the benzyl alcohol to benzaldehyde by the action of bichromate in 80 per cent sulfuric acid.
Benzaldehyde is used not only as an intermediate for the preparation of various triphenylmethane dyes, but to a still greater extent for perfuming almond oil soaps.
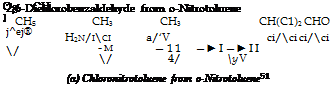
The cheaper varieties of these soaps are adulterated with nitrobenzene (oil of mir — bane); the adulteration can be recognized by the yellow color of the soap.
Chlorination of o-nitrotoluene gives about two-thirds of the 2,6 isomer and one — third of the 2,4-nitrochlorotoluene. 2,4-Dichlorobenzaldehyde, obtained from the latter isomer, yields no valuable dyes, and it is desirable, therefore, to separate the isomeric nitrochlorotoluenes. The separation can be accomplished by careful fractional distillation under reduced pressure.
It is desirable to use, as a chlorination vessel, a relatively tall and narrow glass cylinder (Fig. 5) provided with an inlet tube forchlorine, a thermometer, and a reflux condenser. 5 grams of iron powder and
0. 5 gram of iodine are placed in the cylinder, and chlorine is introduced to form iron iodochloride. After a few minutes, 548 grams (4 moles) of thoroughly dry o-nitrotoluene is added, and a rapid stream of dry chlorine is started. The iron iodochloride goes into solution after a short time, forming a dark brown solution, and the temperature rises rapidly. The reaction flask should be surrounded by a water bath at 50-60°C. to prevent overheating. The addition of chlorine is interrupted when 95 per cent of the calculated amount (131 grams) has been taken up — usually after about 4 hours. Since large amounts of both chlorine and hydrogen chloride remain in solution in the reaction mixture, they must be removed by evacuation before weighing. The dark-colored reaction mixture is washed thoroughly with dilute hydrochloric acid, then with water, and finally freed from acid by washing with sodium hydroxide solution. The mixture is then fractionated under reduced pressure, using an efficient column having a partial condenser, such as that described on page 342, and regulating the partial condenser to give a reflux ratio of 15 or 20 to 1. Unchanged o-nitrotoluene (about 56 to 60 grams) comes over first, then the temperature rises rapidly to 114.6° at a pressure of 11 mm. The first fraction (about 250 grams) is chemically pure
2,6- nitrochlorotoluene (m. p. 37°). Pure 2,4-nitrochlorotoluene (about
90 grains, m. p. also 37°) can be obtained from subsequent fractions by centrifuging in a porcelain centrifuge. The eutectic mixture (about 260 grams), expelled in the centrifuging operation, can be separated into its constituents by repeated fractionation. In plant processes, it is added to the next batch to be purified.
 10 октября, 2015
10 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 