Benzo light grey BL consists mainly of a blue gray dye mixed with a small amount of an orange red brown dye and, in certain cases, with some direct deep black. The presence of a mixture is clearly evident when a little of the dry powder is dusted onto wet filter paper. The blue gray dye can be obtained almost pure by reprecipitation (dissolving in hot water and salting out), leaving a mother liquor which dyes a dull brownish gray.
Reduction. Reduction of the reprecipitated dye with stannous chloride and hydrochloric acid produces a brown solution. Nothing separates out from the hot solution. The reduced solution is therefore electrolyzed directly and then evaporated somewhat. On cooling the solution, a light brown substance, rather sensitive to air, is obtained. This compound gives a condensation product with phenanthrenequi — cone which has an absorption band at 611.0 mp in concentrated sulfuric acid solution. This phenanthrazine is identical with that from 1-amino-y acid. Hence, in benzo light grey BL, acid-coupled у acid is present, undoubtedly as an end component since у acid couples only once. All other reactions also indicate that the reduction product is 1-amino-y acid.
109 Ges. f. Chem. Ind. Basel, Ger. Pat. 120,081 (1901) [Frdl, 6, 865 (1900- 1902)1.
»°M. L. B., Ger. Pat. 142,899 (1903) [Frdl., 7, 466 (1902-1904)1.
1,1 See also Schort and Stewart, J. Chem. Soc., 1929, 553.
"2 Bayer, Ger. Pat. 293,184 (1916) [Frdl., 13, 515 (1916-1921); C. A., 11, 1906 (1917)1.
Further evaporation of the reduction mixture precipitates a second fraction whose properties are not very characteristic. This fraction is not a single compound. It gives no characteristic reaction with ferric chloride, showing the absence of a phenolic hydroxyl group, and it must be, therefore, a naphthylaminesulfonic acid or some other amino compound which gives no condensation product with phenanthrene — quinone.
As a third fraction, a very easily soluble substance is obtained. This material gives a weak blue fluorescence in aqueous or weakly alkaline solution, resembling the fluorescence of naphthylaminesulfonic acids of the type of Freund acid, Laurent acid, or Acid IV. The substance can be diazotized and combined with R salt to produce a dye having two rather unsharp absorption bands at 515.8 and 490.8 m/». These properties indicate that the reduction compound is Acid IV.
If the original dye is reduced with hydrosulfite and the resulting alkaline solution is steam distilled, no base is obtained in the distillate. There is present, therefore, no simple aromatic amine of the benzene series, but only sulfonic acids or naphthylamines.
Since such light fast, direct dyes are usually trisazo dyes, a possible structure is synthesized from 2-naphthylamine-4,8-disulfonic acid, a — naphthylamine, Cleve acid, and у acid (acid coupling).
This compound dyes cotton blue gray from a weakly alkaline bath, the tint being practically identical with that given by purified benzo light grey BL.
|
|
Preparation of the Dye. Acid IV (0.1 mole) is diazotized (like sul — fanilic acid) and to the diazo solution is added a formic acid solution of a-naphthylamine. The coupling reaction is carried out for 24 hours, and the reaction mixture is then heated to boiling. The dye is salted out from the hot solution and reprecipitated from alkaline solution. The precipitated monoazo dye is then diazotized indirectly, and the diazo compound is filtered off and coupled with Cleve acid in the presence of formic acid. After 24 hours (the coupling reaction is slow), the solution is wanned up and made alkaline. The dye is salted out and reprecipitated 4 times until the mother liquor is reddish violet in color. This disazo dye is violet in alkaline solution and blue in acid, and goes directly on cotton. It is diazotized as above, the diazo compound is isolated and coupled in acetic acid solution with Gamma acid (у acid dissolved in soda and acidified with acetic acid). After coupling, the solution is heated to 90°C. and made slightly alkaline with ammonia. The dye is then salted out and filtered off hot.
 23 января, 2016
23 января, 2016  Pokraskin
Pokraskin 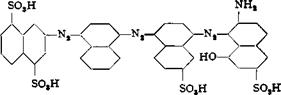
 Опубликовано в рубрике
Опубликовано в рубрике 