(a) Mordant Dyes Alizarin
|
CO CO CO Anthraquinone-2′ 1-Hydroxyanthra — Alizarir sulfonic acid quinone-2-sulfonic acid |
Alizarin (1,2-dihydroxyanthraquinone) is formed by alkali fusion of sodium anthraquinone-2-sulfonate (“silver salt”). The reaction is rather remarkable in that not only is the sulfo group replaced by hydroxyl, but a second hydroxyl is also introduced. The presence of an oxidizing agent has a favorable effect on the reaction.
The alizarin fusion was first undertaken industrially by Caro. In the earlier days, saltpeter was used as the oxidizing agent but this was replaced some seventy years ago by chlorate, following the suggestion of Koch, and today most processess still employ. electrolytic sodium chlorate, which is cheap.
A mixture containing the equivalent of 100 grams of 100 per cent
“silver salt” (page 228), 260 grams of 100 per cent sodium hydroxide, 28 grams of sodium chlorate, and enough water to make a total volume of 670 cc. is heated at 185°C. with continuous stirring in an autoclave. The pressure increases to 5 or 6 atmospheres. After 48 hours, the apparatus is cooled, and a test is made to determine whether the fusion is completed. To this end, 2 cc. of the melt is removed, the alizarin is precipitated with the necessary amount of concentrated hydrochloric acid, and the filtrate is extracted twice with ether. The solution, thus freed
|
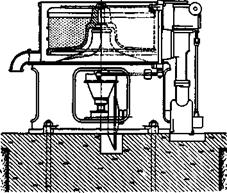
Fig. 33. Centrifuge with bottom discharge. |
from alizarin, is diluted to 15 cc. and examined for fluorescence caused by unchanged silver salt or monohydroxyanthraquinonesulfonic acid. Only a very weak, if any, fluorescence should be visible. If necessary, the mixture is heated for an additional 24 hours at 190°. The melt is then diluted with 2 liters water, the mixture is heated to boiling, and the alizarin is precipitated with 50 per cent sulfuric acid. The dye is filtered off at 50° and washed until the washings are free from salts. The alizarin is not dried since then it does not dye well. The yield is determined by drying a test sample. Usually, the dye is made up to a 20 per cent preparation. About 70 grams of pure alizarin is obtained from 100 grams of pure silver salt.
Technical Observations: Alizarin was the first naturally occurring coloring
matter which was successfully prepared synthetically on a commercial scale. This synthesis was a triumph for the then young coal-tar dye industry, and for a long time alizarin was its most important product. The world production of alizarin (as 100 per cent material) was about 2,800,000 kilograms yearly, of which the Badische A. S.F. supplied 2,000,000 kilograms. In more recent times, the consumption of alizarin has been greatly reduced as a result of the competition of more easily applied red dyes of the azo series, especially some of the equally fast naphthol AS combinations.
|
|
 |
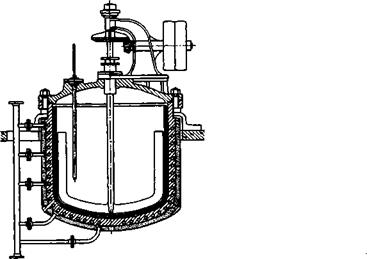
Alizarin fusions are carried out in Frederking apparatus, such as shown in Figure 34, or in similar kettles of the Samesreuther type (Fig. 35a and b). Since the chlorate-containing melt[61] attacks the apparatus, the equipment is always made with an insert of alkali-resistant cast iron which can be replaced easily. There are many variations in these apparatus. The industrial preparations are run in very large batches; in some cases 2000 to 2500 kilograms of 100 per cent alizarin is made at one time in one reaction kettle. The product is made up into either a 20 per cent or a 16 per cent paste. The material is standardized by determining the dry weight and by test dyeing. In plant operations, it is possible to use much less sodium hydroxide, only 110 per cent of the theoretical amount (in our example, only about 40 grams instead of 260 grams). Alizarin, once dried, can easily be regenerated by dissolving it in borax and reprecipitating with acetic or sulfuric acid. Dyes of the alizarin type must be precipitated from boiling solution because of their low solubility. Only in this way can a finely divided product be obtained. A solid preparation of alizarin is prepared for the Orient by mixing the dye with enough starch to make dry lumps which, when placed in boiling water, form a paste suitable for dyeing.
 14 декабря, 2015
14 декабря, 2015  Pokraskin
Pokraskin 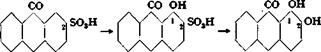
 Опубликовано в рубрике
Опубликовано в рубрике 