(1) Nitrophenoldisulfonic Acid. 94 grams of pure phenol is melted at 50° in an iron sulfonation kettle, and 300 grams of 100 per cent sulfuric acid is added to it, with good stirring, over a period of 10 minutes. The mixture is heated to 100°C. for 1 hour, then cooled to 50°, and 300 grams of oleum containing 60 per cent of SO:? is added. (Only one-half of this quantity of oleum may be used, corresponding to the practice in large scale preparations. It is generally better, in the laboratory, to use the larger amount.) The mixture is again heated at 100° for 1 hour, then cooled in an ice-salt bath to ~5°, and a mixture of 100 grams of 65 per cent nitric acid and 100 grams of 100 per cent sulfuric acid is added dropwise, the temperature being kept between —5 and 0°. The nitration requires about 3 hours, and must not be carried out too rapidly or picric acid will be formed. After 10 hours, the reaction mixture is’ poured into a mixture of 500 grams of ice and 1 liter of water, and the nitrophenoldisulfonic acid is salted out with 300 grams of sodium chloride. The total volume is now about 2 liters. The sodium sulfonate is
precipitated as a light yellow solid which is filtered off after 10 hours and washed on the filter with 200 cc. saturated salt solution. The yield of moist press cake is about 380 to 400 grams.
(2) Reduction. The moist press cake is added in small portions during the course of 30 minutes to a hot (100°) solution of 480 grams of crystalline sodium sulfide (Na2S ♦ 9H20) in about 50 cc. water. The temperature rises to about 108°, and the evaporated water is replaced so that the volume is held constant. The reaction mixture first becomes brown, then it turns reddish brown and a crust of reddish crystals is formed. After an hour, the solution becomes lighter in color. It is heated at 105° for 2.5 hours in all, keeping the volume constant. Hydrochloric acid is now added until the mixture shows a definite and permanent acid reaction to Congo red. It is allowed to stand overnight and is then filtered. The precipitate, consisting of sulfur and the aminophenoldisul — fonic acid, is boiled with 700 cc. water, and the boiling solution is filtered. The colorless filtrate is treated with 120 grams of potassium chloride and cooled. Even at 90°, the acid potassium salt of aminophenoldi — sulfonic acid separates practically completely in a colorless form. It is filtered off after 10 hours and pressed out on the filter. The yield is about 230 grams of dry material.
Salicylic Acid from Phenol
|
|
Salicylic acid is prepared today exclusively by the Kolbe-Schmitt method, in which absolutely dry sodium phenolate is treated with dry carbon dioxide, first at ordinary temperature, then at 125° under a pressure of 4 to 7 atmospheres. The reaction proceeds quantitatively if the salt is completely dry and pulverized to a dust. This fine subdivision is achieved by drying and grinding in a vacuum.
In a stirring autoclave, equipped with a valve for the introduction of carbon dioxide, are placed 94 grams of pure phenol and a solution of 1 mole (40.1 grams of 100 per cent material) of carbon dioxide-free sodium hydroxide in about 100 cc: water. (Sodium hydroxide can be completely freed from carbonate by dissolving in an equal weight of water and allowing the solution to stand for 1 day at 50°. It is then filtered through asbestos and titrated, using phenolphthalein as an indicator.) The solution is evaporated at 100°C. under reduced pressure with constant stirring until no more water is removed. The dry phenolate is then removed from the autoclave and is rapidly pulverized as finely as possible in a previously warmed porcelain dish. It is then immediately returned to the autoclave together with 5 to 10 iron or stone balls of about 14-mm. diameter which serve to pulverize the material still more during stirring in vacuum. The phenolate is heated at 165° in vacuo until it is absolutely dry, which requires 5 to 6 hours. The material is then cooled to 30°, and carbon dioxide is introduced into the apparatus with continuous stirring, adjusting the reducing valve of the cylinder so that the pressure in the autoclave does not exceed 1 atmosphere. After 2 hours, the pressure is slowly increased to 5 atmospheres and the temperature is raised to 125°. After 1 hour more, the introduction tube is removed and the pressure released. After cooling the autoclave, the yellowish, powdery salicylate is dissolved in 400 cc. water and precipitated with 125 grams of 30 per cent hydrochloric acid. Almost pure salicylic acid is precipitated. It is filtered off at 30° and washed with a small amount of water to remove any residual phenol. The product may be purified by distilling with steam superheated to 140°, or by recrystallizing from hot water after precipitating impurities by adding 5 per cent by weight of stannous chloride.[33] The yield of pure, distilled salicylic acid is as high as 125 grams from 94 grams of phenol.
Technical Observations. The industrial apparatus is fashioned after the laboratory equipment, except that it has a very strong stirring mechanism so that it is not necessary to remove the salt from the autoclave for pulverizing. Special stirring mechanisms are also used for this purpose, having arms operating within one another, making the balls unnecessary. Purification of salicylic acid can also be carried out by sublimation in a stream of hot air, yielding a beautiful product which is, however, not entirely pure. The distilled acid always gives better yields of dyes. In large scale preparations, the yields are almost quantitative, amounting to 137 kilograms of salicylic acid from 94 kilograms of phenol.
o-Cresotinic Acid is obtained in a similar manner from o-cresol. In this case, however, the operations must be carried directly through to the end because the sodium salt of o-cresol is spontaneously inflammable. About 20 per cent of the o-cresol is recovered unchanged, and the o-cresotinic acid must be reprecipitated from water. (The material is dissolved in soda, the boiling solution treated with dilute hydrochloric acid, and the product removed by filtering the hot mixture.) In spite of this, o-cresotinic acid is not more expensive than salicylic acid, because the poorer yield is offset by the lower cost of o-cresol.
Salicylic and cresotinic acids are used in the preparation of azo dyes and of tri — phenylmethane dyes of the aurine type (eriochrome azurol B). These acids confer upon the dyes the property of going onto metal mordants, particularly chromium mordants, and also of forming complex compounds, in substance, with suitable metal salts. Salicylic acid is also widely used in the pharmaceutical industry.
The Kolbe-Schmitt reaction is also used in the preparation of the technically very important 2-hydroxyl-3-naphthoic acid from 0-naphthol.
/^Aminosalicylic Acid
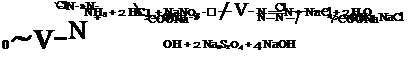
Since salicylic acid is not easily nitrated or nitrosated, p-aminosalicylic acid is usually prepared by reduction of the azo dye from diazotized aniline and salicylic acid, according to the following equations:
![]()
![]()
![]() — OH + 4 Na2SOj
— OH + 4 Na2SOj
COONa
 9 октября, 2015
9 октября, 2015  Pokraskin
Pokraskin 
 Опубликовано в рубрике
Опубликовано в рубрике 