Alkali fusion brings about the replacement of a sulfo group in an aromatic nucleus by hydroxyl, and thus affords a synthesis for numerous technically important phenols and phenol derivatives. Particularly in the naphthalene series, alkali fusion ts one of the most frequently used operations, along with sulfonation, nitration, and reduction.
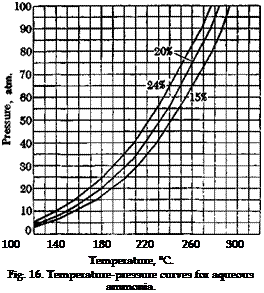
There are two essentially different methods for carrying out alkali fusions in practice. The first method, as in the example given above, uses molten, dry, or almost dry, caustic soda in an open container. The other method employs an aqueous alkali solution, whose concentration may vary between yide limits, and the necessary high temperatures are obtained by working in autoclaves (see temperature-pressure curve, Fig. 15). The second method has the advantage that the reaction can be controlled more accurately, and the optimum conditions for each particular sulfonic acid can be arrived at by a suitable choice of alkali concentration and temperature. In addition, any deleterious action of oxygen at the high temperature is minimized by working in an autoclave. For this reason, purer products and better yields are often obtained in the autoclave reactions, particularly where sensitive substances are involved. Fusion in open kettles is less expensive, however, and is preferable if it gives as good results as can be obtained in an autoclave.
Sodium hydroxide is generally used as die alkali, for reasons of cost. There are cases, however, where good results are obtained only with potassium hydroxide,
which* melts more easily and is more active. Sometimes a mixture of potassium and sodium hydroxides is used because of its still lower melting point.
The reaction of di — and polysulfonic acids can usually be carried out so that the replacement of the sulfo groups by hydroxyls takes place stepwise (partial alkali fusion). Thus, phenol-m sulfonic acid is obtained from benzene-m-disulfonic acid under mild conditions, while resorcinol is formed under more vigorous conditions (see page 144); similarly, naphthalene-1,5-disulfonie acid yields, first, l-naphthol-5-sulfonic acid, then 1,5-dihydroxynaphthalene, both valuable azo dye
|
Fig. 15. Temperature-pressure curves for aqueous sodium hydroxide solutions. |
components. In the naphthalene series, partial alkali fusion attacks the sulfo group in the а-position first. The technically very important 2,5,7-, 2,8,6-, 1,8,4-amino — naphtholsulfonic acids and the 1,8,3,6-, 1,8,4,6-, and 1,8,2,4-aminonaphtholdisulfonic adds are produced in this way from the corresponding naphthylaminedi — and — trisulfonic acids.
Of the side reactions which frequently occur in alkali fusions, the following might be mentioned:
(1) Replacement of the sulfo group by hydrogen instead of by hydroxyl, simultaneously forming sulfate instead of sulfite. This reaction is usually only an unimportant side reaction, but in certain cases it may become the main reaction. Thus, alkali fusion of 2,3-dihydroxynaphthalene-6-sulfonic acid does not give the expected 2,3,6-trihydroxynaphthalene, but instead, 2,3-dihydroxynaphthalene.
(2) The sulfite formed can exert a reducing action when easily reducible groups are present. For this reason, alkali fusion of nitrosulfonic acids rarely works well. Sometimes this redudng action can be counteracted, especially in the anthra — quinone series, by the addition of an oxidizing agent or an alkaline earth hydroxide; in the latter case, the sulfite is precipitated as an insoluble salt and thus rendered harmless.
(3) The molten alkali can have the opposite effect and exert an oxidizing action. Thus, it is well known that anthraquinone-0-sulfonic acid does not yield 0-hydroxyanthraquinone, but alizarin (1,2-dihydroxyanthraquinone). This reaction can be favored by the addition of an oxidizing agent.
(4) Other substituents, in addition to sulfo groups, may be replaced by hydroxyl. This is especially true for amino groups. If this reaction is undesirable, it must be minimized by using mild conditions, avoiding unnecessarily high temperatures.
As mentioned before, halogen atoms (practically only chlorine) as well as sulfo groups can be replaced by hydroxyl by means of alkali fusion. This reaction is accelerated by copper. Whether a given phenol can be prepared better from the sulfonic acid or from the chloro derivative depends on which of the two starting materials is more easily and cheaply made.
Alkali fusion is useful not only for preparing phenols, but also for effecting many condensation reactions, particularly those involving ring closures. Examples of these are the synthesis of indigo from phenylglycine or phenylglycine-o-carboxylic acid, the preparation of indanthrene from 0-aminoanthraquinone, and the preparation of dibenzanthrone from benzanthrone. In order to get good yields in these condensation reactions, it is frequently necessary to exclude all water and also to take up the water formed in the reaction immediately, for example, by means of sodium amide or quicklime. When quicklime is used, the reaction mixture does not melt completely but only sinters (sinter-fusion). The removal of the water formed in the reaction can also be accomplished by working under vacuum or by passing through a stream of some indifferent gas.
6. Derivatives of Chlorobenzene
o — and p-Nitrochlorobenzene
|
|
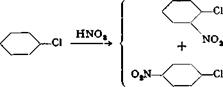
Nitration of chlorobenzene always gives a mixture of about one-third o-nitro- chlorobenzene and two-thirds p-nitrochlorobenzene. The bulk of the para compound crystallizes out on cooling and can be obtained simply by centrifuging. The residual mixture cannot be separated into its constituents by fractional distillation alone because the boiling points lie too close together. However, careful fractionation through an efficient column yields a first fraction enriched in the para isomer, and a last fraction enriched in the ortho isomer. The pure compounds can be isolated from these by cooling and crystallizing, and then centrifuging.[16]* In large scale operations, the noncrystallizing middle fraction, and the eutectic mixture from the centrifuging operation, are put back in the process so that the separation is finally complete. In laboratory operations, one must be satisfied with the isolation of the chief fraction of the para compound and a portion of the ortho isomer.
112 grams (1 mole) of chlorobenzene is heated in an enameled nitrating kettle or a round-bottomed flask on a water bath to 40°C. With vigorous stirring, mixed acid containing 60 grams of sulfuric acid (66° Вё) and 68 grams of nitric acid (sp. gr. 1.52) is added dropwise while the temperature is maintained between 40 and 50°. If the temperature goes much higher, some dinitro compound is formed. Addition of the acid requires about 10 minutes, and stirring is continued for an additional 2 hours, during which time the temperature of the reaction mixture drops slowly. The mixture is now poured out onto ice, while stirring with a glass rod from time to time and keeping the temperature at 0° by addition of more ice. After standing for a long time, the pasty precipitate is filtered off’with suction, washed with cold water, and pressed as dry as possible. It is then centrifuged at about 15° until no more liquid is expelled. The final residue is about 80 grams of practically pure p-nitrochlorobenzene.
The liquid centrifuged out consists of some water and the eutectic mixture consisting of about 30 per cent p — and 70 per cent o-nitro — chlorobenzene. The mixture is separated in a separatory funnel, and the eutectic mixture is washed until it is neutral.
The eutectic mixture is subjected to careful fractional distillation in vacuum, using a column such as that described on page 342. After a small forerun containing water and traces of chlorobenzene, and as soon as the temperature has reached about 105° (10 mm.), the first fraction is collected until about three-fifths of the total volume has come over. This first fraction gives, on cooling and centrifuging at 15°, a few more grams of p-nitrochlorobenzene. The last two-fifths of the total volume is distilled preferably without a column. After cooling, this fraction yields about 10 grams of o-nitrochlorobenzene on centrifuging at 15°,
The liquid eutectic mixtures centrifuged out of both fractions are combined and subjected to a second fractional distillation and crystallization. This process can be repeated as often as appears profitable.
The products obtained are already very pure. If they are desired chemically pure, 1-2 cc. benzene can be sprayed in during the centrifuging. The pure compounds have the following melting and boiling points:
p-Nitrochlorobenzene; m. p., 82.5°C.; b. p. (corr.), 106° at 10 mm., 234° at 727 mm.
o-Nitrochlorobenzene: m. p., 32.5°; b. p. (corr.), 109.5° at 10 mm., 241° at 727 mm.
An accurate melting point curve of mixtures of o — and p-nitrochlorobenzenes has been given by Holleman and de Bruyn.25 From this curve, it is possible to determine directly the composition of any mixture.
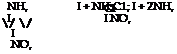 |
||
p — and o-Nitroaniline from p~ and o-Nitrochlorobenzene
 |
One mole of p-nitrochlorobenzene (or o-nitrochlorobenzene) is heated for 10 hours in an autoclave at 170°C. with 10 moles of 25 per cent ammonia. The pressure rises to about 35 atmospheres. After the con-
tents of the autoclave have cooled, the pressure, which is still high, is released. The nitroaniline has separated out in crystalline form, usually containing traces of unchanged nitrochlorobenzene which is removed by recrystallizing the product from boiling water. Since the nitroani-
25 Holleman and de Bruyn, Rec. trav. chim., 19, 192 (1900).
lines are somewhat volatile with steam and strongly poisonous, boiling is done under reflux. The yield is about 95 per cent of the theoretical amount.
Technical Observations. The procedure is different in large scale operations. As soon as the reaction is ended, the excess ammonia is blown out through a condensing system (water), and the autoclave contents, in which all of the nitroaniline remains dissolved at 150°, is put through a strong iron filter press. The nitroaniline is given no opportunity to crystallize out, but is poured directly into cold water, whereupon it crystallizes in finely divided form. This is highly desirable because it can then be diazotized smoothly in the cold.
Analogous procedures are used to prepare the nitroaniline derivatives from l,4-dichloro-5-nitrobenzene and similar compounds. The larger the number of “negative” substituents present, the easier the conversion. The preparation of p-nitrobenzeneo-sulfonic acid (page 99) is another example.
The foregoing procedure has largely replaced the older method for preparing p-nitroaniline (nitration of acetanilide or formanilide, see page 131), although the total cost of the two processes is about equal. The newer procedure is better, however, not only because it yields a product completely free from isomers, but particularly because it supplies an outlet for p-nitrochlorobenzene which is formed in greater quantity than the ortho compound in the nitration of chlorobenzene (page 90). The large demand for the ortho compound for the preparation of o-nitroanisole (o-anisidine, dianisidine, see page 97) resulted in the formation of more p-nitrochlorobenzene than was needed for other purposes.
p-Nitroaniline is used chiefly for the preparation of numerous azo dyes, either in substance or on the fiber (para red). It is also used in preparing p-phenylene — diamine and its derivatives (see next preparation). o-Nitroanilme is also used in the synthesis of azo dyes, but to a much smaller extent It yields a valuable yellow dye for wool when condensed with benzaldehyde and sulfonated.2®
p-Nitrophenyloxamic Acid and p-Aminophenyloxamic Acid27
![]()
![]() NH—CO—COOH NH—CO—COOH
NH—CO—COOH NH—CO—COOH
COOH
COOH
![]() NO,
NO,
In a reaction kettle constructed as shown in Figure 36 (page 333), a mixture of 138 grams (1.0 mole) of p-nitroaniline and 225 grams (2.5 moles) of dry oxalic acid (anhydrous!) is heated with good stirring at 110°C. The two compounds should be finely pulverized and intimately mixed beforehand, and six steel balls of 25 mm. diameter should [17]
be put into the reaction kettle to ensure good mixing. The temperature is raised to 100° over a period of 2 hours, during which time the p — nitroaniline oxalate is formed. Thereafter the mixture is held at 110? for 12 hours with continuous stirring. The water formed is removed with a water pump, some oxalic acid being carried over with it. The end of the reaction is recognized from the fact that the reaction mixture becomes water insoluble. When this point is reached, the crude product is stirred with 1.5 liters of cold water, then filtered off from the
|
|
|
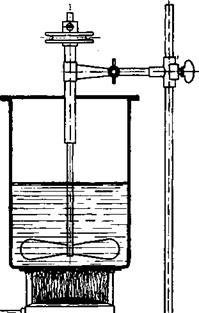
Fig. 17. Apparatus with propeller stirrer for Becnamp-Brimmeyr reductions. |
oxalic acid solution. The precipitate is washed three times on the funnel with 250-cc. portions of cold water. The yield of technically pure p-nitrophenyloxamic acid is about 95 per cent of the theoretical a — mount. The product melts at 205-207°, and after recrystallization from water, at 210°.
Reduction. A mixture of 200 grams of cast iron powder (see pages 75 and 77), 1 liter of water, and 50 cc. concentrated hydrochloric
acid is heated to boiling, and to this is added, over a period of 40 minutes, 0.25 mole (one-fourth of the product obtained above) of p-nitro — phenyloxamic acid. The solution should be kept boiling and vigorously stirred (cf. Fig. 17). The reduction is complete when a drop of the reaction mixture on filter paper shows no color. While the mixture is still boiling, 30 grams of prepared chalk is added and boiling is continued for 30 minutes to convert the product into the calcium salt. 45 grams of soda ash is now added to precipitate all the iron, and the definitely alkaline mixture is filtered. The iron oxide sludge is washed with 200 cc. of hot 2 per cent soda solution. Concentrated hydrochloric acid is added to the clear filtrate until the mixture is just acid to Congo red, whereupon the p-aminophenyloxamic acid separates as a white crystalline precipitate. After cooling, the product is filtered off, washed with cold water, and dried. The yield is about 42 grams, or 93 per cent of the theoretical amount.
p-Aminophenyloxamic acid is an important intermediate in the preparation of polyazo dyes. The oxalic acid residue, in contrast to the acetyl group, is easily removed by hydrolysis, freeing the amino group for diazotization.
p-Nitrophenylhydrazine 28
![]() -N—N + NaCl + 2 HtO Cl
-N—N + NaCl + 2 HtO Cl
![]() -NssN + NasSO, + NaHSO, Cl
-NssN + NasSO, + NaHSO, Cl
![]() Q, N—
Q, N—
-N—NH • SOjK + HC1 + 2H,0 ——- ►
SO, К
Q, N—^ —NH—NHt. HC1 + zKHS04
p-Nitroaniline (34.5 grams, 0.25 mole) is diazotized according to the procedure given on page 246. The diazonium solution is cooled in an ice-salt bath, and soda ash is added to it slowly, with continuous mechanical stirring, until the solution is only very slightly acid to Congo red (about 18-20 grams is required). Care should be exercised
28 Bamberger and Kraus, Ber., 29, 1834 (1896).
that the soda is immediately dispersed so that no portion of the solution becomes alkaline; the temperature should be kept as near to 0°C. as possible, and in no case should it be allowed to rise above 5°. The last traces of free acid are then neutralized with a small amount of sodium bicarbonate. The solution is filtered through a fluted filter to remove a few brown flocks, and the clear, light yellow filtrate is poured with continuous stirring into a solution, cooled to 10°, made by mixing 155 grams (0.55 mole) of sodium bisulfite solution (38° Be) and 21 cc. (0.25 mole) sodium hydroxide solution (40° Be). A clear, intensely greenish yellow solution, neutral to Congo red, is formed. Potassium chloride (120 grams) is now added to precipitate the difficultly soluble
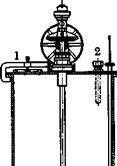
![]() Fig. 18. Reduction kettle with auger — type stirrer: (1) filling port with clamped cover; (2) hole for blow-out tube.
Fig. 18. Reduction kettle with auger — type stirrer: (1) filling port with clamped cover; (2) hole for blow-out tube.
potassium salt of nitrophenylhydrazinedisulfonic acid, and stirring is continued until all of the potassium chloride is dissolved. After standing overnight in a cold place to complete the precipitation, the thick yellow paste is filtered off with suction, pressed out, and washed with a cold 10 per cent potassium chloride solution. The filter cake, which should not weigh more than 170 grams when properly pressed out, is transferred to a porcelain dish and treated with 140 cc. concentrated hydrochloric acid (21° Be), added gradually while stirring with a pestle. Stirring is continued until a completely homogeneous paste is formed, and the mixture is allowed to stand at room temperature for 4 hours. Then it is warmed on a water bath, again stirring thoroughly ivith a pestle, until the yellow, finely crystalline paste is converted to a coarsely crystalline, light red suspension of p-nitrophenylhydrazine hydrochloride. Heating on the water bath is continued for another 15 minutes, during which neither resinification nor the smell of nitroben
zene should be noticeable. The mixture is placed in the cold chest overnight, then filtered with suction and washed with a cold mixture of equal volumes concentrated hydrochloric acid and water. The filter cake is dissolved in 300 cc. lukewarm water and filtered to remove a small residue of brown flocks; then most of the hydrochloric acid is neutralized with 25 cc. 25 per cent ammonia (the solution should still be acid to litmus; otherwise it is acidified with acetic acid). The precipitation is completed by adding a solution of 15 grams of crystalline sodium acetate in 40 cc. water. The mixture is chilled thoroughly, and the orange-red crystals are filtered off with suction. The filtrate should be tested with more acetate to see that no more precipitation occurs. The precipitate is washed with cold water and dried at about 80°C. in a steam heated drying oven.
The yield of product melting at 156-157° (sintering a few degrees lower) is about 30 grams, or 70-80 per cent of the theoretical amount. This product is suitable for most purposes without further treatment. Recrystallization from 20 parts of alcohol gives completely pure p-nitro- phenylhydrazine in the form of slender brown plates with a bluish surface reflection, melting sharply at 157-158°.
o-Nitrophenylhydrazine is prepared in an entirely analogous manner from o-nitroaniline, except that here, in order to separate the potassium salt of the sulfonic acid, it is necessary to make the solution strongly acid with hydrochloric acid after the addition of potassium chloride.29
In this case, the diazotization can be carried out satisfactorily according to the procedure given on page 246 for 3-nitro-4-toluidine, using 100 cc. concentrated hydrochloric acid and 120 g. ice for 0.25 mole o-nitroaniline.
Technical Observations. The nitrophenylhydrazines are used in preparing nitrophenylpyrazolones (page 129) which, after reduction of the nitro group to amino, are valuable components for substantive azo dyes. They can be diazotized and coupled on the fiber, or after treated with formaldehyde.30
p — and o-Nitrophenylhydrazines are important reagents in research for the isolation and identification of aldehydes and ketones. They are especially important in sugar chemistry.
o-Nitroanieole from o-Nitrochlorobenzene
лі лрц
/>о, NaOCH», />o,
128°
28 Muller, Montigel, and Reichstein, Helv. Chim. Acta, 20, 1472 (1937).<
30 See, for example: Chem. Fab. Griesheim-Elektron, Ger. Pat. 278,*871 and 278,872 (1914) [Frdl, 12, 334-339 (1914-1916); C. A., 9, 1847 (1915)h M. L. B„ Ger. Pat. 287,071 (1915) IC. A., 10, 1933 (1916)1; Jordan and Neelmeier, Ger. Pat. 289,350 (1915) [C. A., 10, 2802 (1916)1; M. L. B., Ger. Pat. 296,141 (1916) [Frdl., 13, 527 (1916-1921)1.
In an autoclave of 1-liter capacity, without stirrer, and heated by an oil bath, is placed 600 cc. dry methyl alcohol in which 23 grams of metallic sodium has previously been dissolved (reflux condenser), and to this is added 158 grams of pure o-nitrochlorobenzene (m. p. about 32°C.; b. p. 243°). The autoclave is sealed and the heating is started. The temperature is raised to 120° over a period of 1 hour, held at this point for 3 hours, and finally held at 128° for 1 hour more. The pressure is 8 to 10 atmospheres. At the end of the reaction, the methyl alcohol is blown out through the valve into a good condenser. The recovered methyl alcohol can be used without purification in a subsequent run. The reaction product is removed from the autoclave, washing the latter out with hot water to remove the sodium chloride. The crude product is washed twice with five times its volume of hot water, separated, and distilled in vacuum. The yield is 136 grams, or 88 per cent of the theoretical amount. The product boils at 141° at 15 mm.
Technical Observations. A whole series of alkyl and phenyl ethers in the benzene series (also in the naphthalene and anthraquinone series) can be prepared in an analogous manner. While anhydrous methylate gives satisfactory results in the case of nitroanisole, there are other cases (e. g., dinitroanisole from dinitrochloro — benzene) where it is better to use caustic potash, the small water content having no deleterious effect. Moreover, better results are often obtained if only 90 per cent of the calculated quantity of alkali is used.
An interesting technical example is the preparation of l-amino-2,4-dimethoxy- 5-cMorobenzene according to the following scheme:
![]()
![]()
![]()
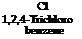
![]()
![]()
![]()
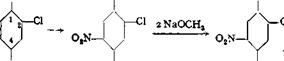 OCH,
OCH,
|
|
|
l-Nitro-2,4,5- trichlorobenzene |
|
,5- l-Nitro-2,4-dimethoxy- zene 5-chlorobenzene |
|
Cl |
|
wnj l-Amino-2,4-dimethoxy- 5-chlorobenzene |
|
OCH. |
|
Cl Naphthol AS-ITR |
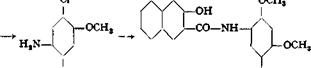
l-Amino-2,4-dimethoxy-5-chlorobenzene is used in preparing the important naphthol AS-ITR, which is used to produce turkey red shades on cotton. The
reactions above are quite similar to those used in the preparation of o-nitroanisole, so it is unnecessary to describe them further.
o-Nitroanisole is used in the preparation of o-anisidine and o-dianisidine, both of which are important bases for azo dye manufacture. The first base is prepared by a Bechamp-Brimmeyr reduction as was described for aniline (page 75). o-Dianisidine is prepared similarly to benzidine (see page 124), converting o-nitro — anisole into the hydrazoanisole by dissolving in alcohol or solvent naphtha and treating with zinc dust and caustic soda, and then subjecting the hydrazoanisole to the “benzidine rearrangement”
The preparation of o-nitroanisole by the methylation of o-nitrophenol is described on page 148.
p-Nitrochlorobenzene-o-sulfonic Acid and p-Nitroaniline-
o-sulfonic Acid from p-Nitrochlorobenzene
|
Cl Cl NH,
NO, NO, NO, |
To a mixture (50°) of 100 grams of p-nitrochlorobenzene and 100 grams of 100 per cent sulfuric acid is added, with stirring, 280 grams of oleum (25 per cent SO3). The mixture is heated at 100-110° until the nitrochlorobenzene has disappeared, then poured into 300 grams of ice and 300 grams of water and salted out with 200 grams of sodium chloride. After 24 hours, the product is filtered off and pressed out, yielding about 280 grams of moist filter cake. The conversion to p-nitroanilinesulfomc acid is accomplished by heating the broken up material with an equal weight of concentrated ammonia (20 per cent NH3) in an autoclave at 150°C. for 8 hours. The pressure is about 6 atmospheres (steel tube manometer!). On cooling, the ammonium salt of the desired sulfonic acid separates in large, hard, ambercolored cubes. The isolated product weighs about 100 grams. In large scale operations, the mother liquor is worked up with chalk to recover the ammonia.
p-Nitroaniline-o-sulfonic acid is used particularly for the preparation of azo dyes; with ^-naphthol, for example, it gives the useful dye, lake red P. Its reduction product, p-phenylenediaminesulfonic acid, is especially useful in the preparation of azine dyes; it is also used, in the form of its monobenzoyl derivative, in preparing azo dyes.
p-Nitrochlorobenzenesulfonic acid is finding increasing application in the preparation of diaminodiphenylaminesulfonic acid as well as aminodiphenylamine — sulfomc acid. These preparations are shown in the following reactions.
 19 сентября, 2015
19 сентября, 2015  Pokraskin
Pokraskin 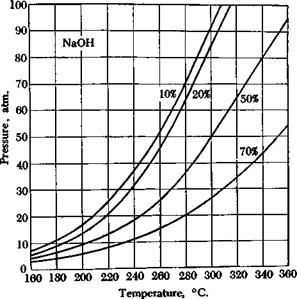
 Опубликовано в рубрике
Опубликовано в рубрике 