![]() NH—CO—CH2—CO—СН,
NH—CO—CH2—CO—СН,
I
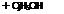 + CH,—CO—СН,-
+ CH,—CO—СН,-
Acetoacetanilide
Acetoacetanilide reacts with NaOH to form:
NH—С—CH* — С—CH,
Л 11 1
О ONa
In the coupling reaction with diazo compounds, the H* is replaced by the —N=N—R groups.
A mixture of 156 grams (1.2 mole) of freshly distilled acetoacetic ester (b. p. 65°C. at 12 mm.), 500 cc. dry toluene, and 0.5 cc. pyridine, is heated to 135° in a flask attached to a downward condenser. To the gently boiling mixture is added, during the course of 5 hours, a mixture of 93 grams (1.0 mole) of freshly distilled aniline, 250 cc. toluene, and 0.5 cc. pyridine. The toluene should distill out’ at about the same rate at which the addition is made. When the addition is completed, the reaction mixture is heated under reflux for 2 hours more. It should be slightly greenish in color, not red. The hot mixture is transferred to a beaker and allowed to cool, whereby pure white acetoacetanilide separates. After 12 hours, the product is filtered off, washed with 50 cc. toluene, and dried at 60°. The yield is about 150 grams, or 85 per cent of the theoretical amount.- In some cases, by-products may be formed in small quantity along with the desired anilide. Their presence is recognizable immediately by failure of the acetoacetanilide to dissolve completely in dilute sodium hydroxide. Pure acetoacetanilide (m. p. 85°) is obtained by filtering the alkaline solution and acidifying the dear filtrate with dilute hydrochloric acid.
Technical Observations. Acetoacetanilide is used in the preparation of important insoluble pigment colors which are known under the trade name, Hansa yellow[28] (see page 266).
9. Derivatives of Benzenesulfonic Acid
Benzene-m-disulfonic Acid SO, H
|
|
SO, H
To 78 grams (1.0 mole) of benzene is added, with good stirring over a period of 2 hours, 250 grams of 20 per cent oleum, the addition being
made at such a rate that the temperature does not rise above 40-45°C. During the next 2 hours, 200 grams of 66 per cent oleum is added, the temperature then rising to about 75°. Finally the mixture is heated at 90° for 1 hour more. The reaction mixture is then poured into 2 liters of water and the hot solution is neutralized by adding about 400 grams of chalk with stirring. The precipitated calcium sulfate is filtered off by suction and washed thoroughly with water. Soda ash (about 100 grams) is added to the hot filtrate until the mixture gives a weak red color with phenolphthalein paper,.the filtrate is evaporated to dryness, and the residue is dried at 130-140°, giving a yield of product of about* 250 grams, or 90 per cent of the theoretical amount.
Benzene-m-disulfonic acid is used in the preparation of phenol-m-sulfonic acid and of resorcinol. Further sulfonation of benzene-m-disulfonic acid to the trisulfonic acid is accomplished by heating the sodium salt for several hours at about 250°, in the presence of mercury, with 66 per cent oleum.
Resorcinol
SO, Na ONa
![]() + 2 NasSOj -f гН,0
+ 2 NasSOj -f гН,0
S03Na ONa
In the tray of a vacuum baking oven (Fig. 30, page 181), 32 grams (0.8 mole) of sodium hydroxide is dissolved in 20 cc. water by heating to 200° C., then 28.2 grams (0.1 mole) of sodium benzene-m-disulfonate (preceding preparation) is added and the mixture is stirred to form a homogeneous mass. The tray is placed in the oven, and the mixture is heated in vacuum, first to 200° to remove the water, then at 320° at a pressure of 12-15 mm. for 6 hours. The resultant cake, after cooling, is dissolved in a small amount of water, and the solution is made strongly acid to Congo red by the addition of concentrated hydrochloric acid and boiled to drive off the S02. The solution is filtered if necessary, and then extracted exhaustively with ether until the water layer no longer gives a test with FeCl3. The ether extract is dried over anhydrous sodium sulfate, the solvent driven off, and the residue distilled in vacuum. At 12 mm., about 0.5 gram of phenol goes over at about 80° (collected separately), then at 156-159°, about 8.5 grams of pure white resorcinol, m. p. 109-110°. The yield is about 75 to 80 per cent of the theoretical amount.
The alkali fusion of benzene-m-disulfonic acid yields resorcinol only in the absence of water. With aqueous sodium hydroxide under pressure, phenol-m-sul — fonic acid is formed, and if the temperature is increased, phenol and decomposition products are formed, but not resorcinol. The formation of some phenol as a byproduct cannot be avoided completely.[29]
The fusion is best carried out in vacuum, suitably in the vacuum baking apparatus used in the sulfonation of amines by the baking process. When the water is evaporated from the melt, the mass foams and, in order to avoid clogging the suction tube, this tube should be sufficiently wide and the tray should not be more than half filled. In order to obtain good yields, it is necessary to use about twice the sodium hydroxide required theoretically, i. e., about 8 moles per mole of sodium benzenedisulfonate.
 3 октября, 2015
3 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 