To 800 grams of 15 per cent oleum is added, over a period of 10 minutes, 192 grams (1.0 mole) of finely powdered /3-naphthylamine sulfate which has been intimately mixed with 1 gram of anhydrous carbonate. The temperature should not be allowed to rise above 50°C. The reaction mixture is now tested for complete solubility in water and soda; monosulfonation is usually complete in 15 minutes. The mixture is cooled to 40°, and 350 grams of 66 per cent oleum is added over a period of 15 minutes with continuous stirring. The sulfonation is continued for 1 day at 55°, then for an additional day at 85°. Under these conditions, the 2,5,7- and 2,1,5-naphthylaminedisulfonic acids, which are formed first, are converted to 2-naphthylamine-l,5,7-trisulfonic acid:
|
|
On completion of the sulfonation (which can be carried out more or less rapidly according to various procedures), the reaction mixture is cooled to 50° C. and poured into a stirred mixture of 800 grams of water and 1100 grams of ice. The final temperature should be about 60°. Pure 2-naphthylamine-6,8-disulfonic acid precipitates and is filtered off after the mixture has been held for 6 hours at 20°. The filter cake weighs about 210 grams (corresponding to 30 grams of NaN02).
In the dye industry, it is customary to express quantities in terms of nitrite equivalents. Since one gram molecule of any given amine requires 69 grams of NaNO[42] (equivalent to 1 mole of nitrous acid) in diazotization, yields of amines are commonly stated as corresponding to x per cent or x grams of nitrite. Thus, if a given quantity of naphthylaminesulfonic acid reacts with 17 grams of nitrite, the quantity is often described as that which is equivalent to 17 grams of nitrite.
Most technical recipes for azo dye preparation call for 1 gram mole or 1 kilogram mole of an amine, since then the quantities of nitrite, acid, and alkali to be used are always the same. Hence, to the technical chemist, the expression: "the yield corresponds to 35 grams of nitrite,” means that the actual yield is about 50 per cent
The filtrate, having a volume of about 2300 cc., is transferred to a dish, heated to boiling (125°), and held at this temperature for 4 hours* (replacing the evaporated water), whereby the 1 sulfo group is split out:
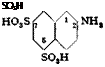 125° HO, s//NHt
125° HO, s//NHt
^ uu
so„H
The solution is held for 2 days at 0° during which the 2,5,7 acid separates. The product, after filtering and pressing, weighs about 170 grams. The mother liquor, which is about 40 per cent sulfuric acid, contains about 22 grams of sulfonic acids per liter, which is not recovered. The 2-naphthylamine-5,7-disulfonic acid corresponds to about 26 grams of NaN02.
The filter cake of the 2,6,8 acid is dissolved in 700 cc. boiling water and treated with 70 grams of salt. The voluminous precipitate of the monosodium salt of the pure acid makes the mixture almost solid. After 12 hours, the mass is broken up and filtered. The crude 2,5,7 acid is dissolved in 850 cc. hot water and reprecipitated in the same way with 85 grams of salt.
The two pure sulfonic acids are distinguishable by their very characteristic fluorescence, which is blue for the 2,6,8 acid and green for the 2,5,7 acid. The latter is clearly observable only with a very pure product and is masked by a small amount of the 2,6,8 acid. Furthermore, the two acids behave differently with an acetic acid solution of diazotized p-nitroaniline. The 2,6,8 acid gives, in dilute solution, only a light yellow coloration due to a diazoamino compound. The 2,5,7 acid, on the other hand, yields a red azo dye immediately. In either case, it is possible to estimate the purity of the product from the depth of color formed. The diazotized 2,6,8 acid couples with R salt to give a difficultly soluble, red azo dye which precipitates even in highly dilute solution and which dissolves on boiling to give a red color. The 2,5,7 acid, under the same conditions, yields an orange red aye which is very easily soluble.
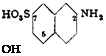
(b) 2-Amino-S-naphthol-6-tulfonic Acid (Gamma Acid)
Alkali fusion of pure naphthylaminedisulfonic acids offers no difficulty, although it is desirable that the material be as free from salts as possible. Pure, dry 2-naphthylamine-6,8-disulfonic acid in an amount equivalent to 35 grams of nitrite (or the corresponding amount of moist material), 220 grams of chlorate-free sodium hydroxide, and 120 grams of water are heated in an autoclave (with stirrer) for 7 hours at 205- 210°C. The pressure increases to 14 atmospheres. After cooling, the pressure is released and the contents of the autoclave are diluted to 1 liter with water (the solution should not smell strongly of ammonia), and made strongly acid with concentrated sulfuric acid. About 250 grams of acid is required. The mixture is filtered after several hours, and the precipitate washed thoroughly with cold water. The у acid is quite insoluble in water. It is pressed out and dried at 100°. The yield is about 105 grams (equal to 95 grams of pure material), or about 80 per cent of the theoretical amount.
у acid is analyzed by titration of its strongly alkaline solution with 1 N diazobenzene solution and by titration with nitrite (diazotization) in very dilute mineral acid solution. (General procedures for such analyses are given in the Analytical Section.). The values obtained in the two titrations should agree within 1 per cent (also in the case of H acid). If the fusion was conducted at too low a temperature, the nitrite titration gives a higher value than the titration with diazotized aniline. The у acid should be at least 91 per cent pure.
(b)
 |
2Amino-5-naphthol-7-sulfonic Acid (J Acid, ito-Gamma Acid)
The procedure is quite similar to that given above for у acid except that it is desirable to use somewhat more water in the fusion mixture — 160 grams instead of 120 grams. The fusion is carried out at a slightly lower temperature, 200-205°C., for 7 hours. The yield of J acid is about equal to that of у acid, i. e., the equivalent of 95 grams of pure material
(about 105 grams of 92 per cent acid, or 82 per cent of the theoretical amount). Thus, the yield is slightly better for the 2,5,7 acid than for the у acid. Side reactions are not prominent in either fusion since the a sulfo group is much more reactive than the one in the /3 position.
The J acid prepared by the above procedure is, in general, sufficiently pure for technical purposes. The product can be purified further by salting out its sodium salt, or by converting it into its difficultly soluble, nicely crystalline zinc salt.
Technical Observations. The small amount of soda admixed with the substance to be sulfonated generates carbon dioxide and thus prevents the formation of hard lumps when the substance is added to the sulfuric acid. The procedure followed in industrial preparations is frequently somewhat different from that given here. Generally, the sulfate or the free /S-naphthylamine is introduced directly into the 40 per cent oleum. The isolation of the different sulfonic acids is relatively easy in the plant, since the separation is more easily accomplished with the larger amounts of material involved. Filtration is usually done in wooden filter presses equipped with nitro filters, and the purified acids or their acid salts can be centrifuged. The various mother liquors, which are too complicated in composition to be resolved in laboratory preparations, are worked up either separately or combined, according to their degree of purity. In this recovery, the liquors are completely neutralized with soda and evaporated in multiple evaporators in vacuum until sodium chloride separates. This is removed by centrifuging, and the mother liquor is then returned to the process. In disulfonations of this sort, part of the substance is always lost. Some of this loss is due to decomposition, and some is caused by the formation of easily soluble sulfones or sulfamiaes which are recognizable by their yellow color.
7 acid and J acid, along with H acid, are the most important aminonaphthol derivatives in the azo dye industry. They are all used in huge quantities in the preparation of wool and cotton dyes. 7 acid is used today in such large amounts that it is manufactured not only from £-naphthylamine, but also from G acid. J acid has become more important recently ana may soon reach the importance of 7 acid, since the azo dyes prepared from it and its numerous derivatives (see Tables XIII and XIV) have very good properties. They are characterized by especially good affinity for cotton, give pure tints, and have, in part, excellent light fastness (see benzo light blue 2GL, page 279 ff.).
The two other methods for the preparation of у acid are described briefly and schematically in the following paragraphs.
 26 октября, 2015
26 октября, 2015  Pokraskin
Pokraskin 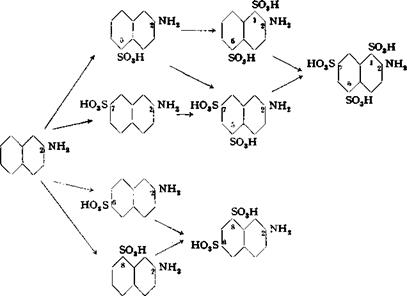
 Опубликовано в рубрике
Опубликовано в рубрике 