Since p-chloro-o-nitrophenol is easily volatile with steam, the reaction vessel must be provided with a tight cover and a reflux condenser (cf. Fig. 11). It is recommended that a very weak vacuum be applied to the end of the reflux condenser so that vapor does not escape from the opening when additions are made, but air is drawn in through it. In spite of this precaution, some of the chloronitrophenol, if it is finely powdered, melts on the spoon, and it is desirable, therefore, to have it in lumps the size of peas. These may be prepared by melting the material in a porcelain dish on a water bath, then allowing it to solidify as a cake which is then broken up. In large scale operations, the nitro compound is introduced as a liquid from a heated container.
The reduction kettle is charged with 50 grams of finely powdered cast iron shavings (see page 77), 200 cc. water, and 25 cc. 2 N hydrochloric acid, and the mixture is heated in a boiling water bath. While stirring vigorously, 34.7 grams (0.2 mole) of 4-chloro-2-nitrophenol is added over a period of 1 to 1.5 hours. Stirring and heating are continued for at least 30 minutes more, after which the chloronitrobenzene which has condensed in the condenser and on the cover and stirrer is washed down into the reaction mixture. The reduction is complete when no more chloronitrobenzene distills into the condenser and its odor has disappeared. Also, a few drops of the reaction mixture on filter paper should be almost colorless and not turned yellow by treatment with sodium hydroxide. The reaction vessel is now opened and 25 cc. 2 N soda solution is added very carefully with stirring to precipitate the dissolved iron, then 25 cc. sodium hydroxide solution (40° Вё) to dissolve the chloroaminophenol.
The iron sludge is filtered off hot and washed carefully with hot water until an acidified test portion of the washings is colored only slightly yellow on addition of nitrite solution. The filtrate, while still warm, is treated with enough concentrated hydrochloric acid (about 25 -28 cc.) to produce a faint acid reaction to litmus, and then the small excess of acid is neutralized by adding a few drops of concentrated sodium acetate solution. Precipitation of glittering plates begins even in the warm solution, and increases on cooling. The precipitation is com-
pleted by adding salt, and allowing the mixture to stand overnight. The solid material is filtered off with suction, washed first with 15 per cent salt solution, then with water, and dried in a steam heated drying oven. The yield is about 26 grams, or 90 per cent of the theoretical amount.
If the reduction has been carried out properly using the pure nitro compound, the product is very light gray in color and melts within a few degrees of the melting point of pure 4 chloro-2 aminophenol (140-141°). It can generally be used without further purification for the preparation of azo dyes. If necessary, the product can be made completely pure by recrystallizing from hot water or by dissolving in hot, dilute hydrochloric acid, filtering, and exactly neutralizing with carbonate solution.
Remarks. The reduction can be effected also with sodium sulfide by the same method given for aminophenoldisulfonic acid (page 153). Isolation of the product is accomplished by neutralizing (with soda) the acid solution which has been filtered to remove sulfur.
4-Chloro-2-aminophenol is used chiefly for the preparation of blue azo dyes which can be after-chromed. Its combinations with chromotropic acid and with acetyl-H acid were the purest chrome blues known before the discovery of the chroming triphenylmethane dyes (eriochrome azurol). The latter dyes are superior in depth and brilliance of color, but are not as fast to light as the azo dyes from 4-chloro.2-aminophenol and chromotropic acid or acetyl-H acid.
7. Derivatives of Nitrobenzene
|
m-Dinitrobenzene from Nitrobenzene
|
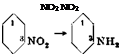
In an iron or glass nitrating vessel equipped with stirrer and thermometer, 70 grams of anhydrous nitric acid (density 1.52), or the equivalent quantity of at least 85 per cent acid, is added with good external cooling to 500 grams of 100 per cent sulfuric acid. To the well stirred acid mixture (which can be replaced by the corresponding quantity of a 30 per cent mixed acid) is added, during the course of 30 minutes, 123 grams of dry, pure nitrobenzene. The reaction vessel is cooled with ice and care is taken that the temperature does not exceed 15-20°C. After all of the nitrobenzene has been added, stirring is continued for 1 hour at room temperature, and then the mixture is warmed to about 35° to redissolve the dinitrobenzene which has separated, and is poured with thorough stirring onto 1 kilogram of ice. The precipitated dinitrobenzene is filtered off and washed with cold water. This crude product is
melted with two 400-cc. portions of water, the first being made distinctly alkaline to litmus by the addition of carbonate.
 |
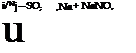 |
The dinitrobenzene obtained in this way contains appreciable amounts of the 1,2 and 1,4 isomers which can be removed easily, since both of these isomers, in aqueous emulsion, react with sodium sulfite and are transformed into the easily soluble nitrobenzenesulfonic acids, whereas the 1,3 isomer is not attacked appreciably under these conditions.
![]() NO.
NO.
+ Na, SO, — + NaNO,
![]() SO, Na
SO, Na
To effect this purification, the crude product is melted in 500 cc. water at about 80°C., and 5 grams of a wetting agent is added (soap, Turkey red oil, Igepon T, Nekal BX, etc.). To the well stirred oily suspension is added, over a period of 30 minutes, 20 grams of crystalline sodium sulfite, and stirring is continued for 2 hours more at 90 to 95°. The mixture becomes dark brown in color as the undesired isomers go into solution. The mixture is allowed to cool to room temperature while stirring is continued, and then the solid precipitate is separated from the mother liquor by filtration. The precipitate is remelted under 500 cc. water and again cooled while stirring. The m-dinitrobenzene, obtained as almost white, small crystals, is dried below 90°. The yield of pure m — dinitrobenzene melting at 90.7°-91.4° (freezing point 90.5-91°) is 140 to 150 grams, or 83 to 90 per cent of the theoretical amount.
Remarks. Dinitrobenzene is strongly poisonous, and therefore the vessels used in remelting and purifying the product should be covered. Alcoholic solutions, especially, can cause cyanosis and protracted eczema. All three isomers are appreciably soluble in water.
Nitration of nitrobenzene always yields, in addition to the m-dinitrobenzene, some (5 to 15 per cent) of the ortho and para isomers, the quantity of these being larger the higher the temperature.[19] It is essential that these isomers be removed in order to obtain, by reduction, m-phenylenediamine which gives good
yields of dyes. m-Phenylenediamine, once prepared, can be separated from its isomers only with difficulty, and only a small amount of o — or p phenylenedi amine in the mete compound is enough to reduce its stability. In addition, the ortho and para isomers, because of their strong reducing action, cause decomposition of diazonium compounds. Hence, if impure m-phenylenediamine is used as a component for azo dyes, persistent foaming is encountered in the coupling reaction because of the generation of nitrogen, and the decomposition products of the diazonium compound contaminate the dye and the yield is decreased (see pages 288 and 292).
m-Dinitrobenzene is used mainly for the preparation of m-nitroaniline and m-phenylenediamine.
 |
m-Nitroaniline from m-Dinitrobenzene
A solution of 110 grams of crystalline sodium sulfide (Na2S • 9H20) in 80 cc. water is treated with hydrogen sulfide until the solution is completely saturated. In this way a clear solution of sodium hydrosulfide (NaSH) is obtained. In a 2-liter glass or iron beaker, another solution is prepared containing 4 grams of Nekal BX (or some other effective emulsifying agent) and 10 grams of ammonium chloride in 420 cc. hot water, and to this solution is added, at 90°C., 84 grams (0.5 mole) of pure m — dinitrobenzene, stirring the mixture vigorously enough to produce a fine emulsion (care! very poisonous vapors). The temperature is allowed to fall to 85° and then, with continued vigorous stirring, the previously prepared hydrosulfide solution is added, during the course of 15 minutes, maintaining the temperature between 80 and 85°. Since heat is given off by the reaction, the flame must be reduced or occasionally removed altogether. When the addition has been completed, stirring is continued for 5 minutes without further heating and then the mixture is cooled to 20° by the addition of ice. Stirring is continued for about 1 hour more at room temperature, and then the m-nitroaniline, which has separated in yellow crystals, is filtered with suction and washed with cold water. The moist filter cake is mixed with 250 cc. water and 80 cc. concentrated hydrochloric acid (technical) and the mixture is boiled until all of the m-nitroaniline has dissolved. The solution is then cooled, causing any residual m-dinitrobenzene, which is quite soluble in hot water, to separate. The solution is filtered to remove undissolved material (chiefly dinitroazoxybenzene), heated, and treated with sufficient ammonia to produce a strongly alkaline reaction. After cooling the solution, the puri
fied m-nitroaniline is filtered off, washed with water, and dried. The yield of the product melting at 110-112° is 62 grams. This material is sufficiently pure for most purposes. It can be made chemically pure by recrystallization from about 4 liters of boiling water, thus removing a very small amount of tarry residue. The recrystallized m-nitroaniline forms golden yellow needles melting at 114°.
The crude product can also be recrystallized from water directly, without previously remelting. If it contains unchanged m-dinitrobenzene, however, some of this impurity will crystallize out with the main product.
 24 сентября, 2015
24 сентября, 2015  Pokraskin
Pokraskin 
 Опубликовано в рубрике
Опубликовано в рубрике 