|
Isomeric dinitroanthraquinones |
QUINIZAMN FROM p-CHLOROPHENOL
(a) 1 £-Dinitroanthraquinone
To a solution of 100 grams of pure anthraquinone (m. p. 278-279°C) in 2000 grams of 100 per cent sulfuric acid is added, at 25° and with good stirring over a period of 30 minutes, 460 grams of mixed acid (230 grams of nitric acid (sp. g. 1,52) and 230 grams of 100 per cent sulfuric acid). The temperature rises to about 80° and 1,5-dinitroanthraquinone begins to separate during the addition of the nitric acid. The mixture is heated for 2 horns at 125° and then cooled. The pure yellow precipitate is filtered off on a sintered glass funnel and washed with 100 cc. 100 per cent sulfuric acid, then with water until the wash water is neutral. The dried product weighs 56 grams which is about 40 per cent of the theoretical amount. The acid mother liquor contains 1,8-, 1,6-, and 1,7- dinitroanthraquinones, which have no technical value.
(b) Reduction to 1,5-Diaminoanthraquinone
If the 1,5-dinitroanthraquinone is to be reduced, best results are obtained if the material from (a) is not dried. A suspension of the moist filter cake in 500 cc. water is heated to 80°C., and a concentrated solution of 350 grams of crystalline sodium sulfide is added. The mixture is stirred vigorously and heated to 100°. The 1,5-dinitroanthraquinone goes into solution forming a green color (alkali-soluble hydroxylamine derivatives ), and after a short time red crystals of 1,5-diaminoanthraquinone separate. After 1 hour, the precipitate is filtered off and washed with water until the washings are colorless. The dried product, which is practically chemically pure 1,5-diaminoanthraquinone, weighs about 40 grams.
27, Quinizarin from p-(,hlor о phenol
Cl OH
СО I CO!
(Y WV-^rY Y4!
/ / / conc — HsSO‘ / //
CO I CO 1
OH OH
Quinizarin is prepared industrially by condensation of phthalic anhydride and p-chlorophenol in the presence of concentrated sulfuric acid and boric acid at a temperature of 160-210°C. The boric acid ester of quinizarin which is formed can be separated by adding ice, and hydrolyzed to quinizarin by warming with water or soda solution. The
crude product must be purified by reprecipitation, recrystallization, or sublimation.
A mixture of 270 cc. concentrated sulfuric acid and 25 grams of boric acid in a 3-necked flask is heated to 50°C. in an oil bath until the boric acid is dissolved. While the temperature is kept at 50°, 96 grams of phthalic anhydride and 26 grams of p-chlorophenol are added alternately in portions, over a period of 1 hour, to the well-stirred acid mixture. The temperature is then raised slowly to 160° and held there for 3 hours. During the course of the next hour, the temperature is raised to 210° and held at this point for 4 hours more. It is essential that overheating be avoided.
The reaction mixture is now diluted to 60° Вё by slowly adding a mixture of 150 grams of concentrated sulfuric acid and 130 cc. water. It is then cooled thoroughly and poured onto ice. The precipitated boric acid ester is filtered off and mixed with 1 liter water. The suspension is boiled for 10 minutes with good stirring, filtered hot, and the precipitate is washed with 1 liter hot water. The mother liquor and wash water are collected separately, since the excess phthalic acid is crystallized from the former (about 40 per cent of the anhydride is recovered as phthalic acid).
The crude quinizarin is purified by stirring the filter cake into a paste with 50 cc. sodium hydroxide solution (50° B6), adding hot water to make 1.5 liters, and heating to boiling by passing in steam. The solution is stirred vigorously and the quinizarin is precipitated as fine crystals by adding concentrated hydrochloric acid slowly. Further purification is effected by boiling the material with 1 liter water containing hydrochloric acid, filtering hot, drying the solid at 80-90°C., and finally crystallizing from chlorobenzene. The yield of purified quinizarin, melting as 194°, is about 35 grams, or 70 per cent of the theoretical amount.
Note. pChlorophenol, which has a strong unpleasant odor, can be replaced in laboratory preparations by an equivalent amount of hydroquinone.
 11 ноября, 2015
11 ноября, 2015  Pokraskin
Pokraskin 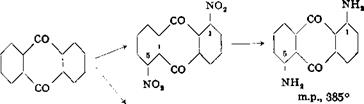
 Опубликовано в рубрике
Опубликовано в рубрике 