White pigments are conveniently classified as either hiding or non-hiding types, depending on their ability to provide opacity. By far the most important white opaque pigment is titanium dioxide (TiO2, C. I. Pigment White 6). It finds widespread use in paints, plastics, printing inks, rubber, paper, synthetic fibres, ceramics and cosmetics. It owes its dominant industrial position to its ability to provide a high degree of opacity and whiteness (maximum light scattering with minimum light absorption) and to its excellent durability and non-toxicity. The pigment is manufactured in two polymorphic forms, rutile and anatase, the former being far more important commercially. The rutile form has a higher refractive index (2.70) than the anatase form (2.55), a feature which is attributed to the particularly compact atomic arrangement in its crystal structure, and it is therefore more opaque. In addition, the rutile form is more durable.
Two processes are used in the manufacture of titanium dioxide pigments: the sulfate process and the chloride process. The chemistry of the sulfate process, the longer established of the two methods, is illustrated schematically in Scheme 9.1. In this process, crude ilmenite ore, which contains titanium dioxide together with substantial quantities of oxides of iron, is digested with concentrated sulfuric acid, giving a solution containing the sulfates of Ti(iv), Fe(iii) and Fe(ii). Treatment of this
|
Scheme 9.1 Sulfate process for the manufacture of TiO |
solution with iron metal then effects reduction of the Fe(iii) ensuring that the iron in solution is exclusively in the Fe(ii) oxidation state. The solution at this stage is then concentrated, thereby depositing crystals of FeSO4-7H2O (copperas), a major by-product of the process which is removed by filtration. Subsequently, in the critical step, the solution is boiled, leading to a precipitate of hydrated titanium dioxide as a result of hydrolysis of the aqueous titanium(iv) sulfate. The hydrated oxide formed is finally calcined at 800-1000 °C to remove water and residual sulfate (as H2SO4) and this leads to the formation of anhydrous titanium dioxide. The sulfate process may be adapted to prepare either the rutile or anatase form of the pigment, by using a ‘seed’ of the appropriate material at the precipitation stage.
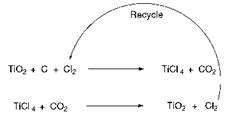
In the more modern chloride process (Scheme 9.2), rutile titanium dioxide ore is initially treated with chlorine in the presence of carbon as a reducing agent at 800-1000 °C to form titanium tetrachloride. After purification by distillation, the tetrachloride is subjected to gas-phase oxidation at 1500 °C with air or oxygen to yield a high purity, fine particle size rutile titanium dioxide pigment. Chlorine is generated at this stage and may be recycled. The two manufacturing processes are of roughly comparable importance on a worldwide basis. However, the chloride process offers certain inherent advantages over the sulfate route. These include suitability for continuous operation, excellent control of pigment properties and fewer by-products, which in the case of the sulfate process can lead to waste disposal problems.
Zinc sulfide (ZnS, C. I. Pigment White 7) and antimony(iii) oxide (Sb2O3, C. I. Pigment White 11) are white hiding pigments which find some specialist applications but their lower refractive indices mean that they are less efficient than TiO2 in producing opacity. White lead (basic lead carbonate, C. I. Pigment White 1), formerly the traditional white hiding pigment, has become virtually obsolete on the grounds of both inferior technical performance and toxicity.
Non-hiding white pigments, sometimes referred to as extenders or
|
Scheme 9.2 Chloride process for the manufacture of TiO |
fillers, are low cost products used in large quantities particularly by the plastics industry. They are white powders of relatively low refractive index and thus they are capable of playing only a minor role in providing opacity. They are, however, used in a variety of other ways. For example, they may be used to modify the flow properties of paints and inks and to modify the mechanical properties and lower the cost of plastics. Commonly used non-hiding white pigments include calcium carbonate (CaCO3), barium sulfate (BaSOJ, talc (hydrated magnesium silicate), china clay (hydrated aluminium silicate) and silica.
 30 ноября, 2015
30 ноября, 2015  Pokraskin
Pokraskin 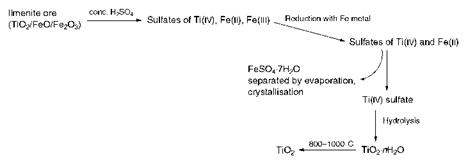
 Опубликовано в рубрике
Опубликовано в рубрике 