The valence-bond (or resonance) approach to bonding in organic molecules is a particularly useful approach to explaining the properties of aromatic compounds. The approach involves postulating a series of organic structures that represent a particular compound in each of which the electrons are localised in bonds between atoms. These structures are referred to as canonical, or resonance, forms. The individual structures do not have a separate existence, but rather each makes a contribution to the overall structure of the molecule, which is considered to be a resonance hybrid of the contributing forms. Arguably the simplest example of resonance is benzene which, to a first approximation, may be considered as a resonance hybrid of the two Kekule structures as shown in Figure 2.7. It was Bury who, in 1935, first highlighted the relationship between
|
Figure 2.7 Valence-bond (resonance) approach to the structure of benzene |
resonance and the colour of a dye, noting that the more resonance structures, of comparable energy, which could be drawn for a particular dye, the more bathochromic were the dyes.
The valence-bond approach may be used to provide a qualitative account of the Xmax values, and hence the hues, of many dyes, particularly those of the donor-acceptor chromogen type. The use of this approach to rationalise differences in colour is illustrated in this section with reference to a series of dyes which may be envisaged as being derived from azobenzene, although in principle the method may be used to account for the colours of a much wider range of chemical classes of dye, including anthraquinones (see Chapter 4), polymethines and nitro dyes.
The valence-bond approach to colour and constitution requires that certain assumptions be made concerning the structures of the electronic ground state and of the electronic first excited state of the dye molecules. Invariably, a dye molecule may be represented as a resonance hybrid of a large number of resonance forms, some of which are ‘neutral’ or normal Kekule-type structures, and some of which involve charge-separation, particularly involving electron release from the donor through to the acceptor groups. For the purpose of explaining the colour of dyes, a first assumption is made that the ground electronic state of the dye most closely resembles the most stable resonance forms, the normal Kekule — type structures. A second assumption is that the first excited state of the dye more closely resembles the less stable, charge-separated forms. The nature of these assumptions will be clarified by a consideration of the examples that follow. As a consequence of Planck’s relationship (AE = hc/X), the wavelength at which the dye absorbs increases (a bathochromic shift) as the difference in energy between the ground state and the first excited state decreases. Essentially the valence-bond approach is used to account for these energy differences. Structural factors, both electronic and steric, which either stabilise or destabilise the first excited state relative to the ground state, are analysed to provide a qualitative explanation of colour. The assumptions which the approach makes concerning the structures of the ground and the first excited states are clearly approximations and cannot be rigorously justified. Nevertheless, evidence that the approximations are reasonable is provided by the fact that the method works well, at least in qualitative terms, in such a large number of cases. There are, however, numerous examples where the approach fails, no doubt due to the inadequacy of the assumptions for those cases.
Table 2.2 shows the 7max values obtained from the UV/visible spectra, recorded in the same solvent, of a series of substituted azobenzenes, which may be considered as the basis of the simplest azo dyes. A number of observations may be made from the data given in Table 2.2. Azobenzene 15a, the simplest aromatic azo compound, gives a Ятах value of 320 nm. This compound is only weakly coloured because its principal absorption is in the UV region. An electron-withdrawing group, such as the nitro group, on its own produces only a weakly bathochromic shift, so that compound 15b (7max 332 nm) is still only weakly coloured. In contrast, an electron-releasing group in the para-position leads to a pronounced bathochromic shift, the magnitude of which increases with increasing electron-releasing power of the group in question. The absorption bands of compounds 15c-e are thus shifted into the visible region (7max 385, 407 and 415 nm respectively) and the compounds are reasonably intense yellows. Very large bathochromic shifts are provided when there is an electron-releasing group in one aromatic ring and an electron-withdrawing group in the other, i. e. a typical donor-acceptor chromogen, as in the case of compound 15f, which is orange-red (Лтах 486 nm).
Valence-bond representations consisting of the most relevant canonical forms contributing towards the structures of compounds 15a-f are illustrated in Figure 2.8. The arguments that follow illustrate how the valence-bond approach may be used to explain colour by rationalising the trends in Лтах values for the compounds given in Table 2.2. Using the approach, it is assumed that the ground state of azobenzene 15a, the parent compound, most closely resembles the more stable traditional
Table 2.2 Xmax Values for a series of substituted azobenzenes (15a-f)
|
|
|
Compound |
X |
Y |
Xmax (nm) in C2H5OH |
|
15a |
H |
H |
320 |
|
15b |
no2 |
H |
332 |
|
15c |
H |
nh2 |
385 |
|
15d |
H |
NMe2 |
407 |
|
15e |
H |
NEt2 |
415 |
|
15f |
no2 |
NEt2 |
486 |
|
Figure 2.8 Valence-bond (resonance) approach to the structure of azobenzenes 15a-f |
Kekule resonance forms, such as structure I. Further, it is assumed that charge-separated resonance forms, such as structure II, make a major contribution to the first excited state of azobenzene. In structure II, the negative charge is accommodated on the electronegative nitrogen atom of the azo group, a reasonably stable situation, but the structure also contains a carbocationic centre, a clear source of instability. The first excited state of azobenzene is therefore rather unstable, i. e. of high energy. In the case of 4-nitroazobenzene (15b), the carbocationic centre is still present as a destabilising feature in the first excited state (structures IV and V). There is, however, a marginal stabilisation of the first excited state in compound 15b as a result of the additional contribution to the first excited state from canonical forms such as structure V, in which charge is delocalised onto the nitro group. Consequently, the energy difference between the ground and first excited states in compound 15b becomes smaller than in azobenzene, 15a, and a small bathochromic shift is observed as a result of the inverse relationship between energy difference and the absorbed wavelength.
The situation is significantly different with the 4-aminoazobenzenes 15c-e. In the charge-separated structures which make the major contribution to the first excited states of these compounds (structure VII), donation of the lone pair from the amino nitrogen atom removes the carbocationic centre, which has a sextet of electrons in its valence shell, and the positive charge becomes accommodated on the nitrogen atom, deriving much greater stability from its full octet of electrons. There is thus a marked stabilisation of the first excited state, lowering its energy and leading to a pronounced bathochromic shift. The electron-releasing inductive effect of the N-alkyl groups in compounds 15d and 15e serves to increase the electron-donor power of the lone pair on the amino nitrogen atom compared with compound 15c thus further stabilising the first excited state and causing a more pronounced bathochromic shift, the magnitude of which increases with increasing electron-releasing power of the alkyl groups. In the case of compound 15f, typical of most aminoazobenzene dyes in which one aromatic ring contains electron — donor groups and the other electron-acceptor groups, the very strong bathochromicity is explained by a first excited state in which there is further stabilisation by charge delocalisation on to the nitro group, as a result of a contribution from structure X.
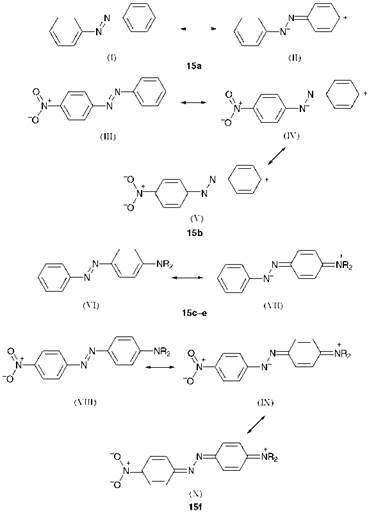
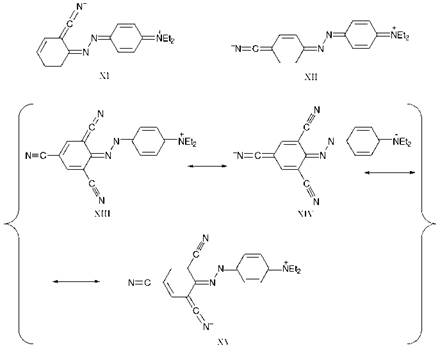
The spectral data for a further group of donor-acceptor aminoazoben — zenes are given in Table 2.3. The valence-bond approach may be used to provide a good qualitative account of the data in the table. Some relevant resonance structures, which may be used to explain the lmax values of cyano compounds 16a-d, are shown in Figure 2.9.
Inspection of the spectral data for the isomeric cyano compounds 16a-c demonstrates that the bathochromicity is most pronounced when the donor and acceptor groups are conjugated with one another, i. e. ortho or para to the azo group, allowing the full mesomeric interaction between the amino and cyano groups in the first excited states to operate, as illustrated for compounds 16a and 16c in structures XI and XII respectively. In contrast, the presence of a cyano group in the meta position gives rise to a less pronounced bathochromic effect (compound 16b) as only the inductive effect of the cyano group is in operation. Increasing the number
Table 2.3 Xmax values for a series of donor-acceptor substituted azobenzenes (16a-f)
|
16 |
|
Compound Acceptor substituents (A) Xmax (nm) in C2H5OH
|
|
Figure 2.9 Resonance forms that make a major contribution to the first excited state of azobenzenes 16a, 16c and 16d |
of electron-withdrawing groups in the acceptor ring increases the bathochromicity, as illustrated by the tricyano compound 16d. The bathochromicity of this dye (Ятах 562 nm) is explained by the extensive stabilisation of the first excited state as a result of resonance involving structures XIIIXV in which the negative charge is accommodated on the
nitrogen atoms of each of the three cyano groups in turn.
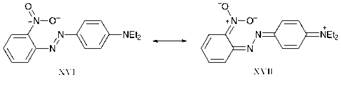
The colour of dyes may be affected by steric as well as electronic effects. For example, a comparison of isomers 16e and 16f shows that an o-nitro group produces a significantly smaller bathochromic shift than a p-nitro group. On the basis of electronic effects alone, delocalisation of charge on to the oxygen atom of the o-nitro group, as illustrated by the valence — bond representation of the structure of compound 16e given in Figure 2.10, might be expected to give rise to a bathochromic shift similar to that given by a p-nitro group. The reason for the differences arises from the fact that while compound 16f is a planar molecule, steric congestion forces compound 16e to adopt a non-planar conformation. This happens because the o-nitro group clashes sterically with the lone pair of electrons on one of the azo nitrogen atoms and is thus forced to rotate out of a planar conformation. It may be envisaged that this rotation takes place about the bond between the carbon atom of the aromatic ring and the nitrogen atom of the NO2 group. As Figure 2.10 shows, the C-N bond in question is of higher bond order in the first excited state (represented approximately by structure XVII), but of low bond order in the ground state (similarly represented by structure XVI). Since rotation about a double bond requires more energy than rotation about a single bond, the first excited state of compound 16e is destabilised relative to its ground state, with a consequent reduction in the bathochromic effect. When it is desirable to have electron-withdrawing substituents in the positions ortho to the azo group to maximise the bathochromicity, cyano groups are commonly preferred. Their linear, rod-like shape minimises the steric clash with the lone pair on the azo nitrogen atoms and allows a more planar conformation to be adopted.
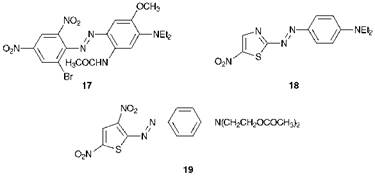
The absorption band of aminoazobenzenes may be shifted to very long wavelengths to produce blue dyes by incorporating a number of electron — withdrawing groups into the acceptor ring and a number of electronreleasing groups into the donor ring. An example of such a dye is compound 17 which gives a Ятах of 608 nm in ethanol as a result of the three electron-withdrawing groups (two nitro, one bromo) in one ring and the three electron-releasing groups (dialkylamino, acylamino and
|
Figure 2.10 Valence-bond (resonance) approach to the structure of compound 16e. |
methoxy) in the other. Alternatively, particularly bathochromic azo dyes that are structurally analogous to the aminoazobenzenes may be obtained by replacement of the carbocyclic acceptor ring with a five-mem — bered aromatic heterocyclic ring. Examples of such dyes are provided by C. I. Disperse Blue 339, 18, a bright blue dye which contains a thiazole ring and gives a Ятах of 590 nm and thiophene derivative 19 which gives a Лтах of 614 nm.
|
|
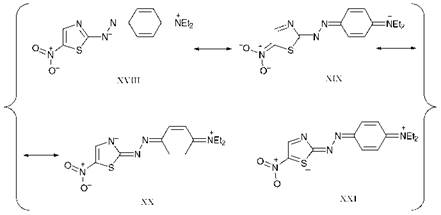
A number of reasons for the particular bathochromicity of heterocyclic azo dyes of this type may be postulated from the valence-bond approach. Some important resonance forms which may contribute to the structure of the first electronic excited state of dye 18 are illustrated in Figure 2.11 The first reason involves a consideration of aromatic character. It is evident that, when the structures which are considered to make a major contribution to the first excited states of azo dyes are compared with those of the respective ground states, electronic excitation causes a loss of aromatic character, i. e. a loss of resonance stabilisation energy. It is generally accepted that five-membered ring heterocyclic systems of this type are less aromatic than benzene derivatives. The loss of resonance stabilisation energy on electronic excitation of dyes such as 18 is therefore less than in the corresponding carbocyclic systems and, as a consequence, the difference in energy between the ground and excited states is less.
Steric effects also play a part in the explanation. For example, the contribution to the first excited state from structure XX (Figure 2.11) illustrates that the heterocyclic nitrogen atom can effectively act as an electron-withdrawing ortho-substituent with no associated steric effect that might otherwise tend to reduce the bathochromicity. Also, in the case of five-membered heterocyclic rings which contain ortho-substituents, such as the o-nitro group in compound 19, the steric interaction between the substituent and the lone pair on the azo nitrogen atoms is less than in the case of six-membered rings, allowing the group to adopt a more
|
Figure 2.11 Resonance forms that make a major contribution to the first excited state of heterocyclic azo dye 18 |
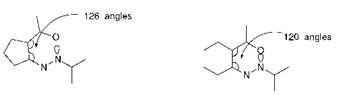
coplanar arrangement and hence maximise the bathochromic effect. This may be explained by a comparison of the geometrical arrangements. As illustrated in Figure 2.12, the azo group makes an angle of 126° with a five-membered ring, compared with a corresponding angle of 120° in the case of a six-membered ring, thus reducing the steric congestion between the oxygen atom of the nitro group and the lone pair on the azo nitrogen atom. A further explanation has been put forward to explain why sulfur heterocycles, in particular, appear in general to provide a pronounced bathochromic effect. It is argued, although it has to be said that there is no real consensus, that there is a contribution to the first excited state from structures such as XXI (Figure 2.11) in which there is valence shell expansion at sulfur. This is possible in principle as a consequence of the availability of vacant 3d-orbitals into which valence electrons may be donated, a situation that is not available in the case of nitrogen and oxygen heterocycles.
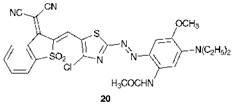
Compound 20 is noteworthy in that it was the first azo dye reported whose absorption band is shifted beyond the visible region into the
|
Figure 2.12 Reduced steric congestion with substituents ortho to the azo group in the case of a five-membered ring compared with a six-membered ring |
near-infrared region of the spectrum, showing a Ятах of 778 nm in dich — loromethane. The extreme bathochromicity of this dye may be explained by a combination of the effects discussed throughout this section, including the extended conjugation, the influence of the thiazole ring and the maximising of both the electron donor (dialkylamino, acylamino, methoxy) and electron accepting (cyano, sulfone, chloro) effects in appropriate parts of the molecule.
It can be seen that the valence-bond (resonance) approach may be used to provide a reasonable qualitative explanation of the 7max values of a
|
|
wide variety of azo dyes. While the use of the approach has been exemplified in this chapter for a series of azo dyes, the argument may be extended successfully to a range of donor-acceptor type chromogens such as carbonyl (see Chapter 4 for an illustration of its application to some anthraquinone dyes), nitro and methine dyes. Nevertheless, the approach does have serious deficiencies. There are a number of examples where wrong predictions are made. For example, the order of bathochromicities of the o-, m — and p-aminoazobenzenes is wrongly predicted (see next section for a further discussion of this observation). Secondly, the approach cannot readily be used to account, even qualitatively, for the intensity of colour by addressing trends in molar extinction coefficients. Finally, and arguably most importantly, the method cannot at the present time be used quantitatively. Quantitative treatment of the light absorption properties of dyes has been made possible by developments in molecular orbital methods, which are discussed in the next section.
 2 сентября, 2015
2 сентября, 2015  Pokraskin
Pokraskin 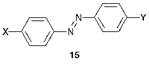
 Опубликовано в рубрике
Опубликовано в рубрике 