The principles of the synthesis of the important chemical classes of dyes from which reactive dyes may be prepared have been discussed in Chapters 3 -6 of this book. This section deals specifically with those aspects of the synthetic sequences which are used to introduce the fibre-reactive group. The starting material for the synthesis of chlorotriazinyl reactive dyes is the highly reactive material cyanuric chloride (2,4,6-trichloro — 1,3,5-triazine), 201. The strategy used in the synthesis of these dyes, as illustrated in Scheme 8.4, involves at appropriate stages of the overall reaction scheme, sequential nucleophilic substitution of the chlorine atoms of compound 201 by reaction with primary amines. As an example, the synthesis of dichlorotriazinyl dye 197a is achieved by formation of the monoazo dye 202 from reaction of diazotised aniline-2-sulfonic acid with H-Acid under alkaline conditions, followed by its condensation with cyanuric chloride 201. Treatment of dye 197a with aniline under appropriate conditions gives the monochlorotriazinyl dye 197b. Since replacement of an electron-withdrawing chlorine atom in compound 201 by an electron-releasing amino group deactivates the product of the reaction towards further nucleophilic substitution, replacement of a subsequent chlorine atom requires more vigorous conditions. This is a useful feature of the chemistry of the process since it facilitates the selectivity of the reaction sequence that leads to mono and dichlorotriazinyl reactive dyes.
|
|
The syntheses of fluorotriazine, trichloropyrimidine and chlorodif — luoropyrimidine dyes are completely analogous, using respectively as starting materials cyanuric fluoride (203), 2,4,5,6-tetrachloropyrimidine (204) and 5-chloro-2,4,6-trifluoropyrimidine (205).
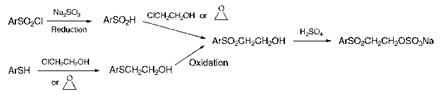
The most commonly employed routes for the preparation of the ^- sulfatoethylsulfone group, which is the essential structural feature of vinylsulfone reactive dyes, are illustrated in Scheme 8.5. One method of synthesis involves, initially, the reduction of an aromatic sulfonyl chloride, for example with sodium sulfite, to the corresponding sulfinic acid. Subsequent condensation with either 2-chloroethanol or ethylene oxide gives the ^-hydroxyethylsulfone, which is converted into its sulfate ester by treatment with concentrated sulfuric acid at 20-30 °C. An alternative route involves treatment of an aromatic thiol with 2-chloroethanol or ethylene oxide to give the ^-hydroxyethylsulfonyl compound which may then be converted by oxidation into the ^-hydroxyethylsulfone.
|
Scheme 8.5 Synthesis of vinylsulfone reactive dyes |
 21 ноября, 2015
21 ноября, 2015  Pokraskin
Pokraskin 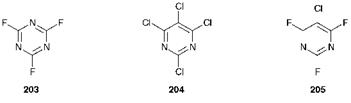
 Опубликовано в рубрике
Опубликовано в рубрике 