The elucidation of the structure of the phthalocyanines followed some pioneering research into the chemistry of the system by Linstead of Imperial College, University of London. The structure that we now recognise was first proposed from the results of analysis of a number of metal phthalocyanines, which provided the molecular formulae, and from an investigation of the products from degradation studies. Finally, Robertson confirmed the structure as a result of one of the classical applications of single crystal X-ray crystallography.
The phthalocyanine system, which may be considered as the tetraaza derivative of tetrabenzoporphin, is planar, consisting of four isoindole units connected by four nitrogen atoms that form together an internal 16-membered ring of alternate carbon and nitrogen atoms. Most phthalocyanines contain a central complexed metal atom, derivatives having been prepared from the majority of the metals in the periodic table. The central metal atom is in a square planar environment. The phthalocyanines are structurally related to the natural pigments chlorophyll (93) and haemin (94), which are porphyrin derivatives. However, unlike these natural colorants, which have poor stability, the phthalocyanines exhibit exceptional stability and they are in fact probably the most stable of all synthetic organic colorants. Copper phthalocyanine, used here as an example, is usually illustrated as structure 92, which contains three benzenoid and one o-quinonoid outer rings. However, it has been established that the molecule is centrosymmetric and this means that structure 92 should be regarded as only one of a large number of resonance forms contributing to the overall molecular structure. The extensive resonance stabilisation of the phthalocyanines may well account for their high stability. The phthalocyanines are aromatic
|
|
molecules, a feature that has been attributed to the 18 я-electrons in the perimeter of the molecules.
The metal phthalocyanines in general show brilliant, intense colours. The UV/visible absorption spectrum of metal-free phthalocyanine (91) in 1-chloronaphthalene shows two absorption bands of similar intensity at 699 and 664 nm. The corresponding spectra of metal phthalocyanines, however, show a single narrow major absorption band, a feature which has been explained by their higher symmetry compared with the metal — free compound and it is the nature of this absorption which gives rise to the brilliance and intensity of their colour. The colours of traditional phthalocyanine dyes and pigments are restricted to blues and greens, although recent years have seen the development of a number of derivatives whose absorption is extended into the near-infrared region of the spectrum. Phthalocyanines are of the polyene rather than the donor- acceptor chromogenic type. As a consequence, the valence-bond (resonance) approach may not be applied so readily to provide an explanation of their colour (Chapter 2). In contrast, the phthalocyanines and structurally related systems have been investigated extensively by molecular orbital methods, including the PPP approach, and these methods generally provide a successful account of the light absorption properties of the system.
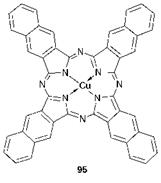
The position of the absorption band of metal phthalocyanines is dependent on the nature of the central metal ion, the substituent pattern on the outer rings and the degree of ring annelation. Among the most extensively investigated phthalocyanines are the complexes of the first transition series metals, iron, cobalt, nickel, copper and zinc. Within this series, the colour is affected little by the nature of the central metal ion; the 1Шах values are to be found in the range 670-685 nm. The most hypsoch — romic of the series of unsubstituted metal phthalocyanines is PtPc (Лшах 652 nm) while the most bathochromic is PbPc (7max 714 nm). Neither of these is of particular interest commercially due either to economic or toxicity considerations, but the bathochromic effect of the vanadyl derivative, VOPc, (7max 701 nm) is of interest from the point of view of extending the absorption range of phthalocyanines. Substituents on the outer aromatic rings almost invariably shift the absorption band to longer wavelengths. Copper hexadecachlorophthalocyanine, for example, absorbs at 720 nm in 1-chloronaphthalene, giving rise to its green colour. There is some considerable interest in phthalocyanines in which the absorption band is extended into the near-infrared region for applications such as optical data storage and security printing (Chapter 10). This may be achieved in a number of ways. For example, the arylthio group causes a much more pronounced bathochromic shift than the halogens and a number of copper polyarylthiophthalocyanine derivatives have been patented for applications which make use of their intense absorption in the near-infrared region of the spectrum. Alternatively, extending the outer ring system by annelation shifts the absorption band bathochromically. Copper naphthalocyanine (95), for example, absorbs at 784 nm, while the corresponding vanadyl derivative gives a 7max value of 817 nm.
|
|
While textile dyes based on phthalocyanines are of rather limited importance, the phthalocyanines provide by far the most important blue and green organic pigments. In particular, copper phthalocyanine (92), C. I. Pigment Blue 15, is by far the most important blue pigment, finding almost universal use as a colorant in a wide range of paint, printing ink and plastics applications. In fact, there is a convincing argument that it is the most important of all organic pigments. It owes this dominant position to its intense brilliant blue colour and excellent technical performance. The pigment exhibits exceptional stability to light, heat, solvents, alkalis, acids and other chemicals. Among the features that demonstrate this high stability are the ability of the material to sublime unchanged at temperatures above 500 °C, and the observation that it dissolves without decomposition in concentrated sulfuric acid, from which solutions it may be recovered. In addition, copper phthalocyanine is a relatively low cost product since, in spite of its structural complexity, its manufacture (see next section) is straightforward, giving high yields from inexpensive, commodity starting materials.
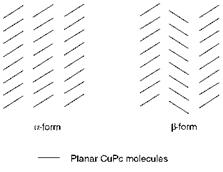
Copper phthalocyanine exhibits polymorphism, the most important crystal phases being the a — and ^-forms, and at least three other forms have been reported. Both the a — and ^-forms are of commercial importance. The two forms exhibit different hues, the a-form being reddish-blue while the ^-form is greenish-blue. The ^-form is the more stable form, particularly towards organic solvents. The a-form has a tendency to convert in the presence of certain solvents into the ^-form with a corresponding change in shade, unless it is stabilised, for example by the incorporation of a single chlorine substituent. ^-CuPc is of particular importance as the cyan pigment used most commonly in printing inks while the a-form is more important in surface coatings and plastics applications. A structural comparison between the a — and ^-phases is shown in Figure 5.1. It has been suggested that in the ‘herring-bone’ arrangement of CuPc molecules in the crystal structure of the ^-form the copper atom at the centre of each molecule is coordinated to nitrogen atoms in adjacent molecules, forming a distorted octahedron, a coordination geometry which is particularly favoured in complexes of copper. No such octahedral coordination is possible in the parallel arrangement of molecules in the crystal structure of a-CuPc, a factor which may contribute to the lower stability of this polymorphic form.
A number of other phthalocyanines are used commercially as pigments. The most important green organic pigments are the halogenated copper phthalocyanines C. I. Pigment Green 7, in which the 16 ring
|
Figure 5.1 The polymorphism of copper phthalocyanine |
hydrogen atoms of the CuPc molecule are replaced virtually completely by chlorine, and C. I. Pigment Green 36, a designation which incorporates a range of bromo — and bromochloro-copper phthalocyanines. The hue of these pigments becomes progressively yellower with increasing bromine substitution. The phthalocyanine greens exhibit the same outstanding colouristic and technical performance as the blue pigments from which they are derived and find equally widespread use in the coloration of paints, printing inks and plastics. Although phthalocyanine complexes have been prepared from virtually every metallic element in the periodic table, only the copper derivatives are of significant commercial importance as pigments, simply because the copper compounds give the best combination of colour and technical properties. However, metal-free phthalocyanine (91) finds some use as a greenish-blue pigment of high stability.
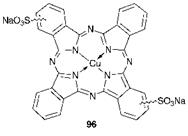
In view of the immense commercial importance of phthalocyanines as pigments, it is perhaps surprising that only a few are of importance as textile dyes. This is primarily due to the size of the molecules; they are too large to allow penetration into many fibres, especially the synthetic fibres polyester and polyacrylonitrile. An example of a phthalocyanine dye which may be used to dye cellulosic substrates such as cotton and paper is C. I. Direct Blue 86 (96), a disulfonated copper phthalocyanine. In addition, a few blue reactive dyes for cotton incorporate the copper phthalocyanine system as the chromophoric unit (Chapter 8).
|
|
 16 октября, 2015
16 октября, 2015  Pokraskin
Pokraskin 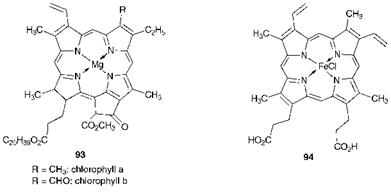
 Опубликовано в рубрике
Опубликовано в рубрике 