The synthesis of metal phthalocyanines requires, essentially, the presence of three components: a phthalic acid derivative, such as phthalic anhydride, phthalimide, phthalonitrile or o-cyanobenzamide, a source of nitrogen (in cases where the phthalic acid derivative does not itself contain sufficient nitrogen) and an appropriate metal derivative. Commonly the reaction requires high temperatures and may be carried out in a high boiling solvent or as a ‘dry bake’ process. In this way, using appropriate starting materials and reaction conditions, virtually the entire range of
|
Scheme 5.1 The phthalic anhydride route to CuPc |
metal phthalocyanines may be prepared. Substituted phthalocyanines are prepared either by using an appropriately substituted phthalic acid derivative as a starting material, or by substitution reactions carried out on the unsubstituted derivatives. Metal-free phthalocyanines are conveniently prepared by subjecting certain labile metal derivatives, such as those of sodium or lithium, to acidic conditions. The method of synthesis is discussed further in this section for the case of copper phthalocyanine, because of the particular importance of this product. Although the structure of copper phthalocyanine is rather complex, its synthesis is remarkably straightforward. It may be prepared in virtually quantitative yield from readily available, low cost starting materials. Two chemically related methods, the phthalic anhydride and phthalonitrile routes, are commonly used for its manufacture. Both involve simultaneous synthesis of the ligand and metal complex formation in a template procedure.
(a) The phthalic anhydride route. In the most commonly encountered version of this method, phthalic anhydride is heated with urea, copper(i) chloride and a catalytic amount of ammonium molybdate in a high — boiling solvent. An outline of the process is given in Scheme 5.1. Urea acts as the source of nitrogen in the process, the carbonyl group of the urea molecule being displaced as carbon dioxide. Mechanistic schemes have been proposed to explain the course of this synthesis but much of the detail remains to be established unequivocally. In essence, phthalic anhydride reacts with urea or products of its decomposition or polymerisation, resulting in progressive replacement of the oxygen atoms by nitrogen and, ultimately, the formation of the key intermediate l-amino-3- iminoisoindoline (97). The presence of ammonium molybdate is essential
|
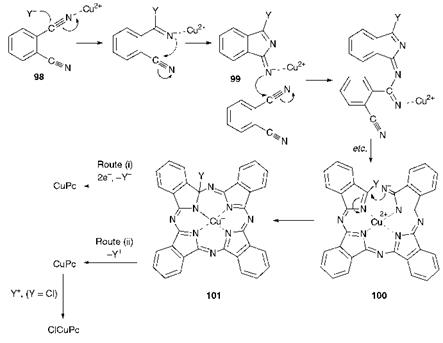
Scheme 5.2 A mechanism for the phthalonitrile route to CuPc |
to catalyse this part of the sequence. Subsequently, this intermediate undergoes a tetramerisation with cyclisation aided by the presence of the copper ion to form copper phthalocyanine.
(b) The phthalonitrile route. In this process, phthalonitrile (98) is heated to around 200 °C with copper metal or a copper salt, with or without a solvent. A mechanism for the phthalonitrile route to copper phthalocyanine has been proposed as illustrated in Scheme 5.2. It is suggested that reaction is initiated by attack by a nucleophile (Y_), most likely the counter-anion associated with the Cu2+ ion, at one of the cyano groups of the phthalonitrile activated by its coordination with the Cu2+ ion. Cyclisation to isoindoline derivative 99 then takes place. Attack by intermediate 99 on a further molecule of phthalonitrile then takes place and, following a series of similar reactions, including a cyclisation step, facilitated by the coordinating role of the Cu2+, intermediate 101 is formed. When copper metal is the reactant, it is proposed that two electrons are transferred from the metal, allowing elimination of Y~ to form copper phthalocyanine [route (i)]. Consequently, the Cu(0) is oxidised to Cu(ii) as required to participate further in the reaction. When a copper(ii) salt is used, it is suggested that Y+ (the chloronium ion in the
|
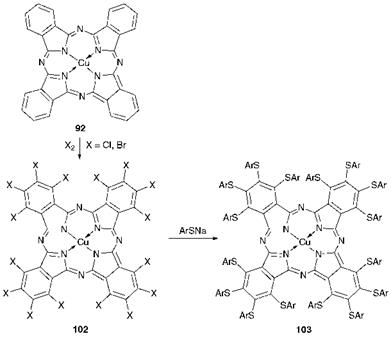
Scheme 5.3 Reactions leading to some substituted CuPc derivatives |
case of CuCl2) is eliminated to form CuPc [route (ii)]. The product in this case, rather than copper phthalocyanine itself, is a monochloro derivative formed by electrophilic attack of Cl+ on the copper phthalocyanine initially formed. Copper monochlorophthalocyanine is important as it exists exclusively in the а-crystal form which, unlike unsubstituted CuPc, is stable to solvents. It has been suggested that the single chlorine atom sterically prevents conversion into the ^-form.
Both the phthalic anhydride and phthalonitrile routes produce a crude blue product, which is of far too large a particle size to be of use as a pigment. The original method developed for particle size reduction used acid pasting, which involves dissolving the crude product in concentrated sulfuric acid, followed by reprecipitation with water. This method gives the а-form of the pigment in a fine particle size form. Mechanical grinding of the crude blue product in the presence of inorganic salts, such as sodium chloride or calcium chloride, produces a mixture of the а — and ^-CuPc which may be converted into pure pigmentary ^-CuPc by careful treatment of this mixture with certain organic solvents. Alternatively, grinding the crude material with inorganic salts in the presence of organic solvents can lead directly to ^-CuPc in a fine particle size form.
Scheme 5.3 shows an outline of some important substitution reactions of copper phthalocyanine. Synthesis of the phthalocyanine green pigments involves the direct exhaustive halogenation of crude copper phthalocyanine blue with chlorine or bromine or an appropriate mixture of the two halogens, depending on the particular product required, at elevated temperatures in a suitable solvent, commonly an AlC13/NaCl melt. These reactions are examples of electrophilic substitution, reflecting the aromatic character of the copper phthalocyanine molecule. The crude green products 102, which are initially formed under these manufacturing conditions, are of large particle size due mainly to a high degree of aggregation. The crude form may be converted into an appropriate pigmentary form either by treatment with suitable organic solvents or by treatment with aqueous surfactant solutions. These processes effect a deaggregation of the product, producing a finer particle size, and in addition they increase the crystallinity of the products. Both of these effects provide a dramatic beneficial effect on the performance of the products as pigments. Treatment of polyhalogenated copper phthalocyanines 102 with thiophenols in the presence of alkali at high temperatures in high-boiling solvents gives the near-infrared absorbing polyarylthio CuPc derivatives 103, as illustrated in Scheme 5.3. This process provides an example of aromatic nucleophilic substitution in the phthalocyanine system. X-ray structural analysis of these arylthio derivatives demonstrates that the sulfur atoms are located in the plane of the CuPc system while the aryl groups are twisted to accommodate the steric congestion.
 16 октября, 2015
16 октября, 2015  Pokraskin
Pokraskin 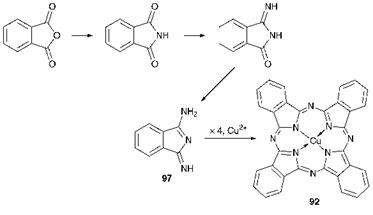
 Опубликовано в рубрике
Опубликовано в рубрике 