Indigo (57) was for many centuries obtained from natural sources. The chemical structure of indigo was first proposed by von Baeyer in 1869, and eleven years later he reported the first successful synthesis, a multistage route starting from o-nitrocinnamic acid. The first successful commercial synthesis of indigo, attributed to Heumann in 1897, is shown in Scheme 4.8. In this classical synthesis, phenylglycine-o-carboxylic acid (79) is converted by fusion with sodium hydroxide at around 200 °C, in the absence of air, into indoxyl-2-carboxylic acid (80). This material readily decarboxylates and oxidises in air to indigo. A much more efficient synthesis, which forms the basis of the manufacturing method in use today, is due originally to Pfleger (1901). In this route, also illustrated in Scheme 4.8, the more readily available starting material, phenylglycine (81) is treated in an alkaline melt of sodium and potassium hydroxides containing sodamide. This process leads directly to indoxyl (82), which undergoes spontaneous oxidative dimerisation in air to indigo. Thioin — digo (60b) is best prepared from o-carboxybenzenethioglycollic acid (83) by a route analogous to Heumann’s indigo synthesis. The final oxidation step in this case uses sulfur rather than oxygen as the oxidising agent.
|
|
|
|
 2 октября, 2015
2 октября, 2015  Pokraskin
Pokraskin 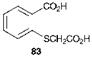
 Опубликовано в рубрике
Опубликовано в рубрике 