A number of methods of synthesis of the quinacridones are reported. Each of these involves several stages, accounting at least in part for the somewhat higher cost of these pigments. The two most important routes to the parent compound are outlined in Scheme 4.9. In both cases, the starting material, diethyl succinylsuccinate (84, 1 mol), which may be prepared by a base-catalysed self-condensation of a succinic acid diester, is condensed with aniline (2 mol) to form the 2,5-diphenylamino — 3,6-dihydroterephthalic acid diester (85). Diester 85 undergoes ring closure at an elevated temperature in a high boiling solvent to give the dihydroquinacridone (86), which is relatively easily oxidised (e. g. with
|
|
|
|
sodium 3-nitrobenzene-1-sulfonate)to the quinacridone 65a. Alternatively, compound 85 may be oxidised to the 2,5-diarylaminoterephthalate diester 87. Base hydrolysis of the diester, followed by ring closure by treatment, for example, with polyphosphoric acid gives the quinacridone.
The formation of a DPP molecule was first reported in 1974 as a minor product in low yield from the reaction of benzonitrile with ethyl bro — moacetate and zinc. A fascinating study by research chemists at Ciba Geigy into the mechanistic pathways involved in the formation of the molecules led to the development of an efficient ‘one-pot’ synthetic procedure to yield DPP pigments from readily available starting materials, as illustrated in Scheme 4.10. The reaction involves the treatment of diethyl succinate (1 mol) with an aromatic cyanide (2 mol) in the presence of a strong base. The reaction proceeds through the intermediate 88, which may be isolated and used to synthesise unsymmetrical derivatives.
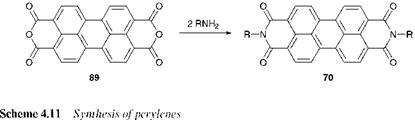
Perylenes (70) are diimides of perylene-3,4,9,10-tetracarboxylic acid, and may be prepared by reaction of the bis-anhydride of this acid, 89 (1 mol) with the appropriate amine (2 mol) in a high-boiling solvent as illustrated in Scheme 4.11. The synthesis of perinones 71 and 72 involves condensation of naphthalene-1,4,5,8-tetracarboxylic acid with benzene — 1,2-diamine in refluxing acetic acid. This affords a mixture of the two isomers, which may be separated by a variety of methods, generally involving their differential solubility in acids and alkalis.
 6 октября, 2015
6 октября, 2015  Pokraskin
Pokraskin 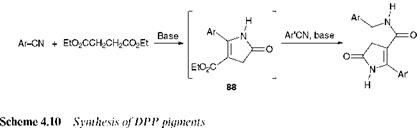
 Опубликовано в рубрике
Опубликовано в рубрике 