Sulfur dyes are a group of low cost dyes used in the coloration of cellulosic fibres. The dyes are fairly small in number although some of the individual products are manufactured in very large quantities. They are capable of providing a wide range of hues although they tend to give rise to rather dull colours, and they are of particular importance as blacks, navy blues, browns and olive greens. In spite of the fact that these products have been known for many years, the chemical structures of sulfur dyes are by no means completely established. They are known to be complex mixtures of polymeric molecular species containing a large proportion of sulfur in the form of sulfide (-S-), disulfide (-S-S-) and polysulfide (-Sn-) links and in heterocyclic rings. C. I. Sulfur Black 1 is by far the most important product in the series. In fact, it may well be the individual dye of all chemical types that has the largest production volume worldwide. It has been suggested that some sulfur dyes are based on the phenothiazonethioanthrone chromophoric system shown in Figure 6.6.
Traditional sulfur dyes are products of high insolubility in water. They are applied to the cellulosic fibres after conversion into a water-soluble leuco form by treatment with an aqueous alkaline solution of sodium sulfide. The chemistry of this process is thought to involve mainly the reduction by the sulfide anion of disulfide (-S-S-) linkages, leading to alkali-soluble thiol (-SH) groups. After application of the leuco form to the fibre, the insoluble polymeric structure of the dye is regenerated by air oxidation and becomes trapped within the fibre. A second group of sulfur dyes are pre-formed leuco dyes which are ‘ready-to-use’ in concentrated aqueous solutions. A third group contains thiosulfate (-S-SO3_ Na+) water-solubilising groups. These dyes, referred to as Bunte salts, are applied to the fibres together with sodium sulfide. During their application the thiosulfate groups are reduced to form dimeric and polymeric species as a result of disulfide bond formation. The particular advantage of sulfur dyes, as a class of dyes for cellulosic fibres, is that they provide reasonable technical performance at low cost. Unfortunately sulfur dyes present some environmental problems, largely associated with sulfide residues which remain in the dyehouse effluent and this feature may cause their use to decline in years to come.
|
Figure 6.6 The phenothiazonethioanthrone chromophoric system proposed as a constituent of some sulfur dyes |
|
|
The manufacture of sulfur dyes involves sulfurisation processes, the chemistry of which remains rather mysterious and may arguably be considered still to be in the realms of alchemy! The processes involve heating elemental sulfur or sodium polysulfide, or both, with aromatic amines, phenols or aminophenols. These reactions may be carried out either as a dry bake process at temperatures between 180 and 350 °C or in solvents such as water or aliphatic alcohols at reflux or at even higher temperatures under pressure. C. I. Sulphur Black 1, for example, is prepared by heating 2,4-dinitrophenol with sodium polysulfide.
 23 октября, 2015
23 октября, 2015  Pokraskin
Pokraskin 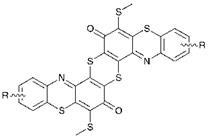
 Опубликовано в рубрике
Опубликовано в рубрике 