In the case of monoazo dyes and pigments, the strategy for synthesis is straightforward, involving appropriate selection of diazo and coupling components, and choice of reaction conditions in accordance with the chemical principles presented in the previous two sections of this chapter. In the case of azo colorants containing more than one azo group, the situation is more complex and it becomes even more critical that the synthetic strategy and reaction conditions are selected carefully to ensure that a pure product is obtained in high yield. A system, in common usage as a systematic treatment of the possible synthetic strategies leading to polyazo compounds, has been proposed by the Society of Dyers and Colourists (SDC). The system has undoubted merit as a method of classification, although rigorous justification of some of the symbolism used may be questioned. To describe the strategies, it is appropriate at this stage to define of the nature of the various reacting species as follows:
A: primary aromatic Amine: i. e. a normal diazo component;
D: primary aromatic Diamine: i. e. a tetrazo component;
E: coupling component capable of reaction with one diazonium ion:
an End component;
Z: coupling component capable of reaction with more than one diazonium ion;
M: coupling component containing a primary aromatic amino group
which, after an azo coupling reaction, may be diazotised and used in a second azo coupling: a Middle component.
Synthesis of Monoazo Dyes and Pigments
The synthesis of monoazo dyes and pigments is represented by the symbolism
A ^ E
In using this terminology, it should be emphasised that the arrow has the meaning ‘diazotised and then coupled with’ rather than its usual meaning in organic reaction sequences. Thus, for example, the first stage in the synthesis of C. I. Disperse Orange 25, 39, is the diazotisation of 4-nit — roaniline, using sodium nitrite and aqueous hydrochloric acid at temperatures less than 5 °C. The diazonium salt thus formed is reacted with N-ethyl-N-^-cyanoethylaniline under weakly acidic conditions since the coupling component is a tertiary aromatic amine.
|
|
Synthesis of Disazo Dyes and Pigments
The situation becomes more complex when two separate diazotisations and azo couplings are required. Four separate strategies leading to disazo colorants may be identified, using the commonly accepted SDC terminology and symbolism, exemplified as follows as strategies (a)-(d).
(a) A1 Z — A2
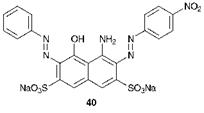
In this the first strategy, two primary aromatic amines (Ai and A2) are diazotised and reacted separately under appropriate pH conditions and in an appropriate sequence with a coupling component which has two available coupling positions. As an example, the synthesis of C. I. Acid Black 1,40, a bluish-black dye commonly used to dye wool, is as follows.
4- Nitroaniline is diazotised under the usual conditions and the dia — zonium salt reacted in the first coupling reaction under weakly acidic conditions with H-Acid, 33, under which conditions azo coupling is directed into the ring containing the amino group. Secondly, aniline is diazotised and the resulting diazonium salt reacted with the monoazo intermediate to form the disazo dye. For the second coupling, alkaline
|
|
conditions are used since a phenolic coupling component is involved. In general, reactions of this type are carried out in this sequence because of the good solubility of the monoazo intermediate in alkali and the improved selectivity of the process when carried out in this way.
(b) E1 — D E2
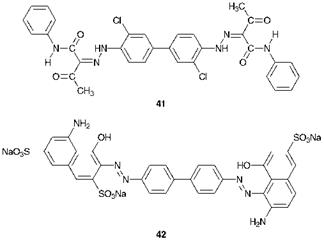
In this strategy a primary aromatic diamine is diazotised twice (tet — razotised) and coupled separately with two coupling components (E1 and E2). In the case of C. I. Pigment Yellow 12, 41, which is an important bright yellow pigment used extensively in printing inks, the product is symmetrical (a bisketohydrazone). This greatly simplifies the synthetic procedure since the two coupling reactions may be carried out simultaneously. 3,3′-Dichlorobenzidine(1 mol) is tetrazotised (bisdiazotised) and coupled under weakly acidic to neutral conditions with acetoacetanilide (38, 2 mol) to give the product directly. When an unsymmetrical product is required, a much more careful approach to the synthesis is essential to ensure that the unsymmetrical product is not contaminated by quantities of the two possible symmetrical products. One approach that may be used involves the diazotisation, by careful choice of conditions, of one amino group of the diamine D followed by coupling with the first component E1. The monoazo intermediate formed contains the second amino group, which may be diazotised followed by coupling with component E2. Alternatively, the synthesis may be achieved by tetrazotisation of the diamine followed by two sequential azo coupling reactions with careful selection of pH conditions to control the outcome of the reactions. In the case of C. I. Direct Blue 2,42, for example, the synthesis could start, in principle, with the tetrazotisation of benzidine (4,4′-diaminobiphenyl). The resulting tetrazonium salt is first coupled with y-Acid (35) under acidic conditions and then the monoazo intermediate is reacted with H-Acid (33) under alkaline conditions. This particular example, by way of illustration, uses benzidine as the tetrazo component. Formerly, benzidine was an important tetrazo component, particularly for the manufacture of direct dyes. However, the compound has for many years now been recognised as a potent human carcinogen and its use in colour
|
|
manufacture has long since ceased in the developed world (see Chapter 11).
(c) A M E
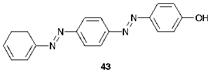
This third strategy in azo colorant synthesis makes use of the feature that a primary aromatic amine has the potential to be used both as a coupling component and as a diazo component. As an example, in the synthesis of C. I. Disperse Yellow 23, 43, aniline is first diazotised and the resulting diazonium salt reacted under weakly acidic conditions with aniline to give 4-aminoazobenzene. A practical complication with this stage is the inefficiency of the coupling reaction with aniline, and the formation of side-products due to an ^-coupling reaction. In an improved method, aniline is first reacted with formaldehyde and sodium bisulfite to form the methyl-®-sulfonate derivative (ArNHCH2SO3Na) which couples readily. After coupling, the labile methyl-®-sulfonate group may be removed easily by acid hydrolysis to give 4-aminoazobenzene. This amine is then diazotised and the resulting diazonium salt is reacted with phenol under alkaline conditions to give the disazo compound 43.
|
|
|
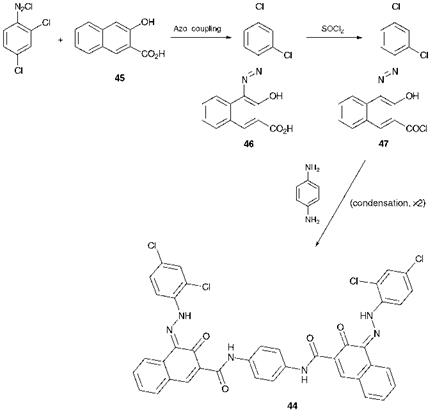
Scheme 3.8 Synthesis of a disazo condensation pigment |
(d) A1 Z-X-Z — A2
The products from the fourth strategy are structurally not dissimilar from those obtained by the strategy given in (a). However, in this strategy the disazo colorant is synthesised by linking together two molecules of a monoazo derivative by some chemical means. As an example, the synthesis of C. I. Pigment Red 166,44, a disazo condensation pigment which exists structurally as a bisketohydrazone, is shown in Scheme 3.8. The monoazo compound 46 containing a carboxylic acid group is prepared by an azo coupling reaction with 3-hydroxy-2-naphthoic acid (45) as the coupling component. The acid 46 is then converted into the acid chloride 47, followed by a condensation reaction between the acid chloride (2 mol) and p-phenylenediamine (1 mol) to form compound 44. In principle, a simpler and more cost-effective route using strategy (a), involving azo coupling of the diazonium salt (2 mol) with the appropriate bifunctional coupling component (1 mol), might be proposed. In practice, this route fails because the monoazo derivative formed from the first azo coupling reaction is so insoluble that the second coupling reaction cannot take place.
 13 сентября, 2015
13 сентября, 2015  Pokraskin
Pokraskin 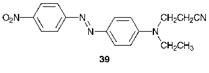
 Опубликовано в рубрике
Опубликовано в рубрике 