The phthalocyanines represent without doubt the most important chro — mophoric system developed during the 20th century. Historically, the most important event was probably their accidental discovery around 1928 by a dye manufacturing company in Scotland. However, there is little doubt that researchers before this had isolated phthalocyanines, but the significance of their observations was not fully recognised. In 1907, Braun and Tcherniac were engaged in a study of the chemistry of o — cyanobenzamide (90) and discovered that when this compound was heated a trace amount of a blue substance was obtained. This compound undoubtedly was metal-free phthalocyanine (91). In 1927, de Diesbach and co-workers reported that when 1,2-dibromobenzene was treated with copper(i) cyanide in boiling quinoline for eight hours, a blue product was obtained in reasonable yield. This was almost certainly the first preparation of copper phthalocyanine (CuPc, 92). They obtained the molecular formula of the compound from elemental analysis and noted its remarkable stability to alkali, concentrated acids and heat but were unable to propose a structure. In 1928, in the manufacture of phthalimide by Scottish Dyes (later to become part of ICI) from the reaction of phthalic anhydride with ammonia in a glass-lined reactor, the formation of a blue impurity was observed in certain production batches. This contaminant was isolated as a dark blue, insoluble crystalline substance. Ultimately, the compound proved to be iron phthalocyanine (FePc), the source of the iron being the wall of the reactor which became exposed due to a flaw in the glass lining. An independent synthesis involving passing ammonia gas through molten phthalic anhydride in the presence of iron filings confirmed the findings. Following this discovery, the colour manufacturing industry was quick to recognise the unique properties of the compounds and to exploit their commercial potential. The phthalocyanines have emerged as one of the most extensively studied
|
|
classes of compounds, because of their intense, bright colours, their high stability and their unique molecular structure.
 15 октября, 2015
15 октября, 2015  Pokraskin
Pokraskin 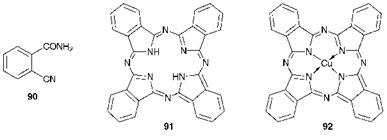
 Опубликовано в рубрике
Опубликовано в рубрике 