Photochromism is generally regarded as the reversible interchange of a single compound between two molecular states, which show different absorption spectra when activated by light. Most commonly, reversible photochromism follows the general principles illustrated in Scheme 10.3 in which structure A (usually colourless) is converted by irradiation with light of a certain wavelength (usually in the UV region) into structure B which is coloured. The reverse reaction takes place either thermally when the light source is removed or by irradiation with light of a different
hv-1
Structure A — Structure В
A or hv2
(colourless) (coloured)
Scheme 10.3 General scheme for reversible photochromism
wavelength. Colour changes due to exposure to light have been known for many years. The term phototropy was formerly used to refer to the change of colour of dyes on textile fabrics when exposed to light, which has always been considered as undesirable even if it is reversible. This effect was particularly evident in some simple aminoazobenzene derivatives used as the first generation of disperse dyes applied to cellulose acetate. The effect was attributed to the photo-induced conversion of the trans isomer of the azo dye into its cis isomer and the associated colour change.
The modern generation of photochromic dyes are used in sun-screening applications, such as sunglasses and car sunroofs, optical data storage, photoresponsive polymers and for a variety of novelty effects. One of the most important groups of photochromic dyes are the spirooxazines. Spirooxazine 247 is non-planar and colourless. On exposure to light, compound 247 ring opens to the violet-blue merocyanine structure 248 (Scheme 10.4). When the light source is removed, it reverts thermally to the more stable ring-closed structure 247. The spirooxazines offer the advantage of good fatigue resistance and relative ease of synthesis.
|
Scheme 10.4 Reversible photochromism of spirooxazine 247 involving interconversion with the merocyanine form 248 |
 19 января, 2016
19 января, 2016  Pokraskin
Pokraskin 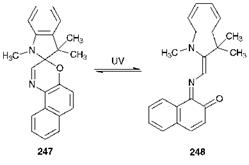
 Опубликовано в рубрике
Опубликовано в рубрике 