Metal complex formation has been a prominent feature of textile dyeing from very early times, since it was recognised that the technical performance, including fastness to washing and light, of many natural dyes could be enhanced by treatment with certain metal ions, a process known as mordanting. Mordant dyeing is still used to a certain extent today, although it is restricted mainly to the complexing of certain azo dyes on wool with chromium(iii) (see Chapter 7). There are basically two types of metal complex azo dyes: those in which the azo group is coordinated to the metal (medially metallised) and those in which it is not (terminally metallised). The discussion in this section is restricted to the former type which are by far the most important commercially.
The most important metal complex azo dyes are formed from the reaction of transition metal ions with ligands in which the ortho positions adjacent to the azo group contain groups capable of coordinating with the metal ion. The most important group in this respect is the hydroxy (-OH) group, although carboxy (-CO2-) and amino (-NH-) groups can also be used. The most important transition metals used commercially to form metal complex azo dyes are copper(ii), cobalt(iii) and, especially, chromium(iii). The reaction of an o, o’-dihydroxyazo compound, which acts as a tridentate ligand, with chromium(iii) is illustrated in Figure 3.5. The azo group is capable of coordination to the metal ion through only one of the two azo nitrogen atoms, utilising its lone pair in bonding. While transition metal complex azo chemistry has proved to be of considerable importance in textile dyes, curiously, and perhaps rather surprisingly, it has provided very limited success in commercial organic pigments, although other types of metal complexes, notably phthalocyanines, are of immense significance in this respect (see Chapters 5 and 9).
|
Figure 3.5 Complex formation between an o, o’-dihydroxyazo compound and chro — mium( iii ) |
1:1 Copper complexes of azo dyes are used widely in both reactive dyes (Chapter 8) and direct dyes (Chapter 7) for cotton. In these dyes, the copper complexes adopt a four-coordinate square planar geometry, with the three coordinating sites of the dye occupying three corners of the square and the fourth occupied by a monodentate ligand, commonly water (Figure 3.6). The most important cobalt and chromium complexes of azo dyes adopt six-coordinate octahedral geometry, with the six positions occupied by coordination with two tridentate azo dye ligands. These products are referred to as 2:1 premetallised dyes and they are of considerable importance in dyeing protein fibres such as wool (Chapter 7).
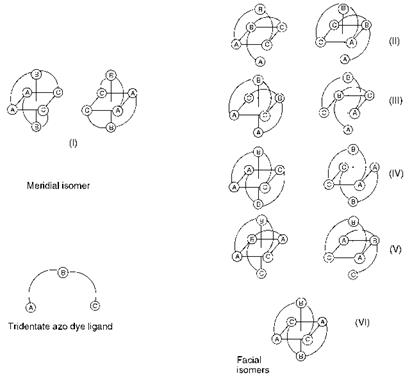
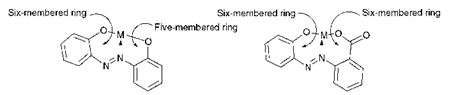
The 2:1 octahedral complexes present many opportunities for isomerism. The isomerism in a number of metal complex azo dyes has been characterised using a variety of techniques, including 1H and 15N NMR spectroscopy and X-ray crystallography, and in some cases isomers have been separated chromatographically. The geometrical isomerism of some metal complex azo dyes is illustrated schematically in Figure 3.7. There are a number of geometrical isomers possible: one meridial (mer) isomer (I), in which the two dye molecules are mutually perpendicular, and five facial fac) (II-VI) isomers in which the molecules are parallel. The meridial arrangement is favoured by complexes of o, o’-dihydroxyazo systems that contain a 5:6 chelate ring system, while the facial arrangement is favoured in o-carboxy-o’-hydroxyazo systems in which there is a 6:6 chelate ring system (Figure 3.8). Because of their asymmetry, the geometrical isomers, apart from (VI) which is centrosymmetric, also give rise to pairs of optically-active enantiomers. Other types of isomerism in azo metal complexes which have been identified include positional isomerism as a result of the possibility of coordination to either (but not both) of the azo nitrogen atoms, and isomerism as a result of different states of hybridisation (sp2 or sp3) of the azo nitrogen atoms.
There are a number of particular technical advantages associated with the formation of coloured metal complexes. Commonly, the transition metal complexes of a coloured organic ligand exhibit lightfastness which is significantly better than that of the free ligand. An explanation that has been offered for this effect is that coordination with a transition metal ion
|
|
Figure 3.6 Square planar azo copper complex
|
Figure 3.7 Isomerism in 2:1 octahedral metal complex azo dyes |
|
Figure 3.8 Chelate ring formation in metal complex azo dyes |
reduces the electron density at the chromophore, which in turn leads to improved resistance to photochemical oxidation. Other effects which may have a part to play in the enhanced lightfastness of transition metal complexes are steric protection of the chromophore towards degrading influences and the ability of transition metal ions to quench excited states that otherwise might undergo photochemical decomposition. In addition, the larger size of the metal complex molecules compared with the free ligand generally gives rise to improved washfastness properties in textile dyes as a result of stronger interactions with the fibre. On the other hand, the colours of the metal complexes are almost invariably duller than those of the azo dye ligand, a feature that limits their usefulness. The reduction the brightness of the colour which accompanies metal complex formation is due to a broadening of the visible absorption band. There are a number of possible reasons for this effect. Broadening may due to the presence of a number of isomers, each with a slightly different absorption band. Alternatively, it may be due to overlap of the absorption band associated with the n-n transitions of the ligand with those arising from metal ion d-d transitions or from ligand-metal charge transfer transitions.
 15 сентября, 2015
15 сентября, 2015  Pokraskin
Pokraskin 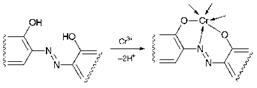

 Опубликовано в рубрике
Опубликовано в рубрике 