Lasers are devices which provide an intense, continuous source of ‘inphase’ radiation. The term laser is an acronym referring to light amplification by stimulated emission of radiation. Traditional laser technology utilises a variety of inorganic materials to produce the required emission. Several different types of inorganic laser have been developed to emit either in the ultraviolet region, the visible region or infrared region of the
|
Figure 10.4 Organic pigments used in colour filters for flat-screen displays |
electromagnetic spectrum. These inorganic lasers are commonly low cost and robust devices, but they suffer from the disadvantage that they emit at only a few selected wavelengths, and in very narrow bands. An example of a commonly encountered inorganic laser is the gallium-arsenic diode laser, which emits in the near-infrared region at around 780 nm. In contrast, dye lasers, which are based on specific fluorescent dyes dissolved in an appropriate solvent, emit a broad band of wavelengths, thus offering the advantage of tunability through a wide range of wavelengths. The applications of dye lasers include communications technology, microsurgery, spectroscopy, photochemistry, studies of reaction kinetics, isotope separation and microanalysis.
The principles of the function of the fluorescent dye in a dye laser may be explained with reference to the mechanism of fluorescence which is described in Chapter 2 (see Figure 2.6). Having absorbed a quantum of light, the dye molecule is promoted from its ground state, S0, to its first excited state, Si*. Lasing occurs when incident radiation interacts with the dye molecule in its excited state, thereby causing (stimulating) the molecule to decay to the ground state by emission of radiation (fluorescence). For the lasing effect to occur, a situation has to be brought about in which the dye molecules exist predominantly in the excited state. This population inversion is achieved by ‘pumping’ the system with an appropriate source of energy, generally a powerful inorganic laser. In
contrast to spontaneous emission, the stimulated emission of radiation from dye lasers is strictly coherent (same phase and polarisation) and is of high intensity.
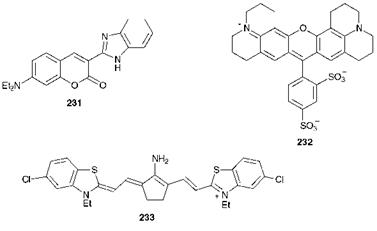
There are a number of general requirements for laser dyes. Strong absorption at the excitation wavelength is clearly required but there should be minimal absorption at the lasing wavelength, so that there is as little overlap as possible between absorption and emission spectra. Additional significant requirements are a high quantum yield (0.5-1.0), a short fluorescence lifetime (5-10 ns), low absorption in the first excited state at the pumping and lasing wavelengths, low probability of intersystem crossing to the triplet state and good photochemical stability. By appropriate dye selection it is possible to produce coherent light of any wavelength from 320 to 1200 nm. Examples of laser dyes are given in Figure 10.5. For shorter wavelengths (up to ca. 470 nm), aromatic hydrocarbons, such as anthracenes and polyphenyls, and fluorescent brightening agent-type materials such as stilbenes, oxazoles, coumarins and car — bostyrils are most commonly used. Benzimidazolylcoumarins such as compound 231 are suitable for use in the 470-550 nm range, while rhodamines, such as compound 232, together with some related xanthene derivatives, are of prime importance in the 510-700 nm region of the visible spectrum. For dye lasers operating at longer wavelengths into the near-infrared region, oxazines and polymethine dyes are most important. For example, polymethine dye 233 provides a lasing maximum at 950 nm.
|
|
 7 января, 2016
7 января, 2016  Pokraskin
Pokraskin 
 Опубликовано в рубрике
Опубликовано в рубрике 