Indigo (57), the parent system of this group of colorants, is one of the oldest known natural dyes (see Chapter 1). The naturally occurring
material is indican, the colourless glucoside of indoxyl. When subjected to a fermentation process, free indoxyl (58) is generated by enzymic hydrolysis and this compound undergoes oxidation in air to indigo. Indigo was formerly made in this way in India, Indonesia and China from the indigo plant (Indigofera tinctoria) and in Europe from dyers’ woad (Isatis tinc- toria) although nowadays, indigo is made purely synthetically. The synthetic routes are outlined later in this chapter.
|
|
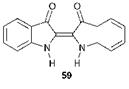
The structure of indigo was first proposed by von Baeyer, although he initially suggested that it had the (Z)- (or cis) configuration 59. An X-ray crystal structure determination carried out by Posner in 1926 confirmed that the molecule in fact exists as the (E)- (or trans) isomer 57. Although hundreds of indigoid derivatives have been synthesised and evaluated as dyes over the years, relatively few of these have achieved commercial importance and indeed the parent compound remains by far the most important member of the class. Indigo is applied to fabrics as a vat dye, imparting an attractive blue colour and good wet fastness properties. It provides only moderate lightfastness, but it shows the feature of fading without changing colour (i. e. on tone). By far its most important use is to dye jeans and other denim articles, which slowly fade to paler shades of blue, a key fashion feature. In fact, its lasting success as a commercial dye will be heavily dependent on the continuation of this popular textile fashion trend.
|
|
A question which has intrigued colour chemists for years is why indigo, a relatively small molecule, absorbs at such long wavelengths. The colour of indigo depends crucially on its environment. It is known that, in the vapour phase, the only situation in which it approaches a monomolecular state, indigo is red. In solution, indigo exhibits pronounced positive solvatochromism; in non-polar solvents it is violet, while in polar solvents it is blue. In the solid state, and when applied to fabric as a vat dye, it is
blue. X-ray structure analysis of the molecule has demonstrated that, in the solid state, the molecules are highly aggregated by intermodular hydrogen bonding, each indigo molecule being attached to four others. It is clear that the intermodular hydrogen bonding is a major factor in providing the bathochromic shift of colour. It also explains its low solubility and relatively high melting point (390-392 °C). It is generally accepted that the basic structural unit responsible for the colour ofindigo is as illustrated in Figure 4.4. It consists of two electron donor groups (NH) and two electron acceptor groups (C-O), ‘cross-conjugated’ through an ethene bridge, the so-called the H-chromophore, and this gives rise to a remarkably bathochromic absorption. The molecule has been the subject of numerous studies using quantum mechanical calculations and these are in agreement with the H-chromophore explanation.
 |
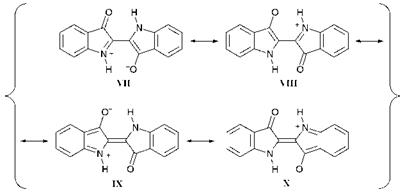
UV/visible spectral data for some indigo derivatives, which illustrate the nature of the substituent effects, are given in Table 4.2. A valence — bond (resonance) explanation has been suggested for the bathochromic- ity of indigo, invoking important resonance contributions to the first excited state from charge separated structures VII-X, in each of which charge is accommodated in a stable location, as illustrated in Figure 4.5. Resonance structures VII and VIII are particularly stable due to the retention of aromatic character of both benzene rings. The effect of
Figure 4.4 The H-chromophore unit of indigo
Table 4.2 UV/visible spectral data (lmax values in nm) for solutions of some symmetrical di-substituted indigo derivatives 57 and 57a-d in 1,2-dichloroethane.
 Substituent
Substituent
H 57,620 nm 57,620nm
NO2 57a, 580 nm 57b, 635 nm
OCH3 57c, 645 nm 57d, 570 nm
|
Figure 4.5 Valence-bond (resonance) approach to indigo |
substituents on the colour of indigo may be explained using this approach. For example, an electron-releasing group in the 5- (or 7)-posi — tions, which are ortho or para respectively to the NH group stabilise the first excited state by increasing the electron density on the nitrogen atom and a bathochromic shift results. In contrast, an electron-withdrawing group at these positions destabilises the first excited state by increasing the positive charge at an already electron-deficient nitrogen atom, causing a hypsochromic shift. For substituents at the 4- and 6-positions, which are in conjugation with the electron-withdrawing carbonyl group, the situation is reversed. The results of PPP-MO calculations are found, in this case, to support the valence-bond explanation.
A range of other indigoid systems, represented by the general structure 60 in which one or both of the NH groups are replaced by other heteroatoms capable of donating a lone pair of electrons, are known. These include the yellow oxindigo (60a, X = Y = O), the red thioindigo (60b, X = Y = S), and the violet selenoindigo (60c, X = Y = Se), together with mixed derivatives such as compound 60d (X = NH, Y = S). In this group, thioindigo (60b) is a useful red vat dye and some of its halogenated derivatives are used as pigments, but the others are of limited commercial significance. Indirubin (61) is a red positional isomer of indigo that is occasionally encountered as an impurity in natural indigo.
 22 сентября, 2015
22 сентября, 2015  Pokraskin
Pokraskin 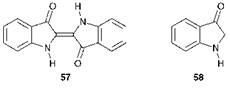
 Опубликовано в рубрике
Опубликовано в рубрике 